Tailoring Exercise for the Aging Brain: Sex-Based Differences in Alzheimer’s, Parkinson’s, and Cognitive Protection
Exercise Reduces Neurodegenerative Disease Risk Through Multiple Mechanisms: Across Alzheimer’s disease (AD), Parkinson’s disease (PD), and other age-related disorders, physical activity lowers risk and slows progression through coordinated effects on autophagy, oxidative stress, vascular remodeling, protein clearance, and neuroimmune balance. Epidemiological data show that exercise reduces AD risk by 45%, while a sedentary lifestyle increases it by 53%. These population findings mirror mechanistic evidence that regular movement enhances neuronal resilience, limits proteotoxic stress, and suppresses chronic inflammation—key drivers of neurodegeneration.
Women Often Derive Greater Cognitive and Structural Brain Benefits From Exercise: Randomized trials and large cohort studies reveal that women—especially postmenopausal women—frequently experience stronger cognitive improvements from aerobic exercise than men. In mild cognitive impairment, 6 months of exercise at 75–85% heart-rate reserve improved executive function more in women, and a meta-analysis of 33,816 adults found that physical activity offered greater cognitive protection for women over 1–12 years. Higher daily step counts also correlate with larger hippocampal volumes in older women, suggesting enhanced neuroplasticity.
Women Show Stronger Irisin Responses, Driving Distinct Neuroprotection: Irisin, a muscle-derived exerkine that crosses the blood–brain barrier, promotes BDNF expression, supports synaptic plasticity, and reduces amyloid accumulation. Irisin-deficient animals display impaired memory and elevated Aβ, while irisin supplementation reverses these effects. High-intensity endurance exercise raises irisin more in women, producing larger acute spikes during maximal cycling tests. This heightened irisin-BDNF signaling may help explain why women often show greater cognitive gains and amyloid reductions from aerobic training.
Estrogen–BDNF Interactions Shape Sex Differences in Brain Aging and Exercise Response: BDNF supports neurogenesis, synaptic strengthening, and non-amyloidogenic APP processing. Because the BDNF gene contains an estrogen response element, estrogen directly boosts BDNF expression. After menopause, BDNF levels decline in women—but not in men—contributing to elevated AD vulnerability. Aerobic exercise and irisin-mediated BDNF signaling appear to partially compensate for this postmenopausal decline, making physical activity especially important for maintaining cognitive resilience in women.
Antioxidant Responses Differ by Sex, With Men Showing Broader Gains: Long-term exercise enhances Nrf2-driven antioxidant defenses, and Nrf2 is the body’s master antioxidant switch that activates genes protecting cells from oxidative damage. In a cohort of 120 adults, physically active men improved buffering of all three reactive species (peroxyl, hydroxyl, and peroxynitrite), while active women improved only against peroxynitrite. Additionally, miR-153, which suppresses Nrf2 signaling, decreased only in active men, indicating that men may gain broader antioxidant protection from exercise.
Amyloid and Tau Responses to Exercise Show Clear Sex-Specific Patterns: Women typically exhibit higher baseline Aβ levels, but exercise substantially reduces their amyloid burden. In one cohort, physically active women saw Aβ fall to 8.94 ng/mg, compared to 12.69 ng/mg in sedentary women, normalizing levels to those of men. Meanwhile, men may exhibit greater improvements in Tau-related pathology and immune-driven protein clearance. These differences illustrate that amyloid and Tau pathways are modulated by exercise in sex-specific ways.
Personalized Exercise Strategies May Optimize Neuroprotection for Each Sex: Although aerobic and resistance exercise benefit both sexes, emerging findings suggest that optimal modalities differ. Women may benefit most from high-intensity endurance exercise that strongly activates the irisin–BDNF axis, while men may benefit from training that enhances antioxidant defenses, Tau clearance, and TLR2 modulation. Meeting or exceeding 150 minutes per week of moderate aerobic activity is strongly associated with reduced neurodegenerative risk across studies. As research evolves, sex-informed exercise prescriptions may better preserve cognitive function across the lifespan.
Introduction
Studies show that from 1990 to 2021, the number of healthy years lost to neurological diseases increased by about 20%, impacting over 40% of people worldwide. This remarkable loss of vitality is only expected to grow globally as the population is aging, leading to the rise of age-related neurodegenerative disorders. These age-associated neurodegenerative disorders, such as Alzheimer’s Disease (AD) and Parkinson’s Disease (PD), represent a class of debilitating conditions marked by the gradual deterioration of neurons, resulting in progressive declines in cognitive abilities, motor functions, and emotional regulation. [1, 2]
As the prevalence of these disorders rises, questions remain: how do we combat this loss of vitality and reduce the likelihood of an age-related neurodegenerative disorder? The answer to these questions is nuanced, as there is currently no cohesive molecular mechanism of disease that fully explains the neuron loss seen in any of the above conditions. Nonetheless, evidence suggests that genetic variants, harmful protein accumulation, oxidative stress, gut-brain axis interactions, impaired autophagy, and neuroinflammation are all factors contributing to the development of neurodegenerative disease. [3]
Although the precise causes of AD, PD, and related disorders remain elusive, one consistent insight has emerged: lifestyle interventions can meaningfully alter the trajectory of brain aging. One such lifestyle intervention is exercise, which is known to reduce the risk of neurodegenerative disorders and stabilize neurological dysfunction in both men and women. In this research review, Dr. Samantha Behunin, PhD, will explore how exercise exerts these neuroprotective effects and how understanding these mechanisms may help preserve brain health. She will also examine how the type, intensity, and benefits of exercise may differ by gender—and what these differences reveal about the interplay between biological sex, hormones, and the aging brain.
Physical Activity as a Preventive and Therapeutic Intervention
Physical activity serves as a notable intervention with the potential to influence neurodegenerative disease risk. For example, physical activity has been demonstrated to reduce the likelihood of developing Alzheimer’s Disease by 45%, while a sedentary lifestyle may increase this risk by 53% [10]. Additionally, moderate exercise is known to modulate oxidative stress and autophagy in neural cells, potentially mitigating the accumulation of proteins linked to neurodegenerative pathology [11].
An important consideration is identifying which forms of exercise are most effective at preventing or treating specific neurological disorders. Santiago and Potashkin evaluated existing research on exercise regimens that lower the risk of AD and PD (Table 1 and Table 2, respectively). As seen from these tables, no single “best regimen” has emerged. Studies demonstrate that both aerobic and resistance exercise have beneficial effects in cases of AD and PD. For Parkinson’s Disease, cognitive improvements are more often linked to moderate or vigorous physical activity rather than to low-intensity workouts. [10]
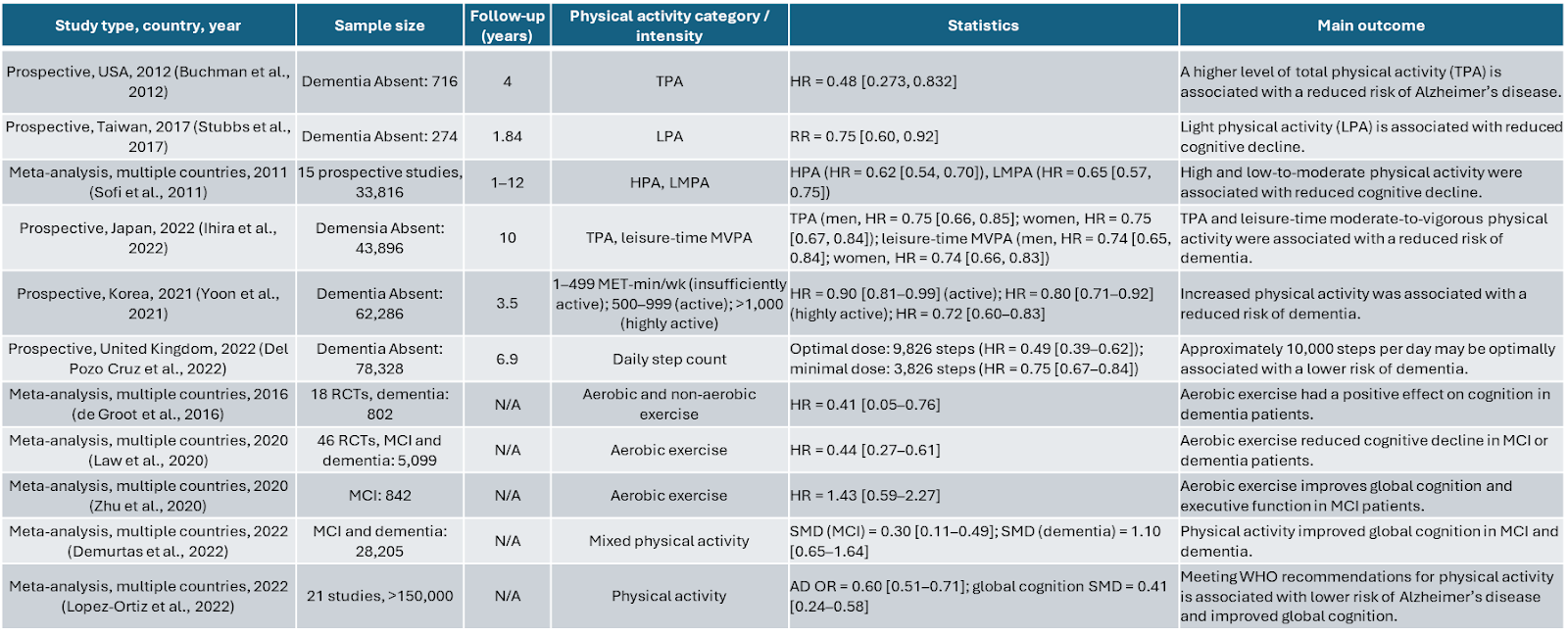
Table 1: Research Studies of Physical Activity and Exercise in AD. PA, physical activity; LPA, light physical activity; TPA, total physical activity; LMPA, low to moderate physical activity; MVPA, moderate to vigorous physical activity; OR, odds ratio; HR, hazard ratio; SMD, standardized mean difference; MET, metabolic equivalent of task; RR, risk ratio; N.A, not applicable[10]
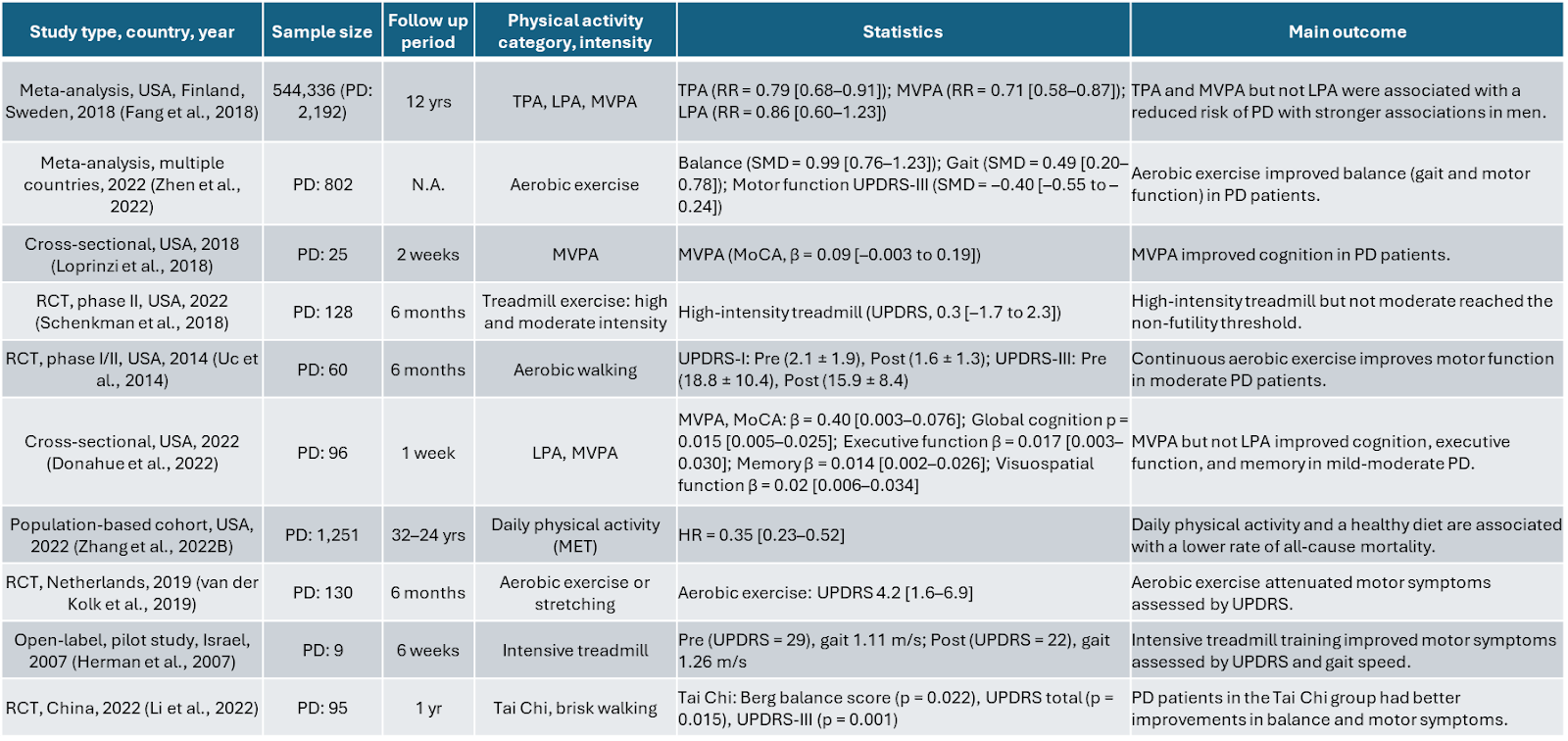
Table 2: Research Studies of Physical Activity and Exercise in PD. PA, physical activity, PD, Parkinson's disease, LPA, light physical activity, TPA, total physical activity, LMPA, low to moderate physical activity, MVPA, moderate to vigorous physical activity, OR, odds ratio, HR, hazard ratio, SMD, standardized mean difference, MET, metabolic equivalent of task, MoCA, Montreal Cognitive Assessment, UPDRS, Unified Parkinson's Disease Rating Scale, RCT, randomized controlled trial, RR, risk ratio, N.A, not applicable.[10]
Exercise Benefits on Neurodegenerative Disease for Women Vs Men
Existing human studies assessing the impact of exercise on neurological disorders exhibit substantial diversity. Variability in study design encompasses factors such as the type and duration of exercise, the inclusion of mixed neurological disease types, age, comorbidities, and sex. As a result, it remains challenging to recommend optimal interventions tailored to individual patient profiles. To address this issue, researchers have begun investigating sex-based differences in the neurological benefits conferred by exercise. This is particularly important as susceptibility to, and progression of, neurodegenerative diseases differ between men and women. For example, women have a higher incidence of AD and multiple sclerosis, whereas men are at increased risk for developing PD and ALS. [11]
Several human studies have indicated possible sex-based differences in neurological responses to exercise. For instance, among individuals with mild cognitive impairment (MCI), aerobic exercise (either treadmill running, cycling or, using the elliptical trainer at 75-85% of heart rate reserve for 45-60 minutes a day for four days a week for over a period of 6 months) was found to improve executive function more in women than men [11, 12].
Furthermore, a meta-analysis of 33,816 individuals without dementia, followed for 1-12 years, concluded that physical activity is associated with protection against cognitive decline. In this meta-analysis, three studies examining sex differences found that physical activity may provide greater cognitive protection for women than for men.
Another meta-analysis reported that females over 55 showed a greater cognitive response to aerobic training than age-matched males [14]. Moreover, exercise has also been linked to the maintenance of brain volume, particularly in regions associated with neurodegenerative disease. Higher levels of physical activity —for example, walking 10,000 steps daily or completing three 10-minute sessions of 1,000 steps daily—were correlated with larger hippocampal volumes in older women than in age-matched men [15].
The differences seen in these studies may arise from sex-specific physiological responses to exercise. By understanding these sex-specific responses, researchers can better define personalized exercise prescriptions to mitigate neurodegenerative disease risk and support cognitive resilience. While not exhaustive of all neurological benefits of exercise, the remainder of this review will highlight emerging evidence that biological sex influences how the brain responds to physical activity, and that optimizing exercise for men and women may be key to extending healthy brain aging.
Exerkines - Powerful Modulators of Systemic Benefits
When we exercise, our bodies release a suite of signaling molecules known as exerkines. These compounds—ranging from myokines and cytokines to nucleic acids, lipids, and metabolites—originate in tissues such as muscle, liver, fat, and nervous system. Exerkines travel through the body, acting on targets that include muscle, blood vessels, bone, fat, immune cells, and the brain itself [16]. Put simply, exercise induces a systemic shift in physiology with exerkines orchestrating changes that bolster both immune defenses and neurological health (Figure 1). This signaling network helps explain why exercise is so broadly beneficial, reaching far beyond muscles to influence the very systems that sustain cognitive and physical vitality.
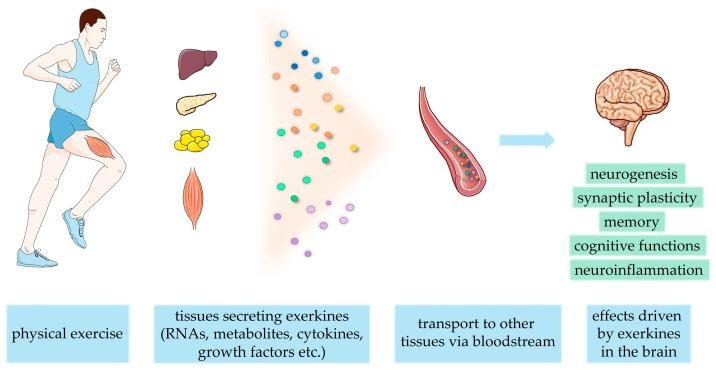
Figure 1: Exercise releases exerkines from multiple tissues, such as the liver, pancreas, adipose, and muscle tissue, to be transported in the blood to target tissues. Some of these exerkines cross the Blood-Brain Barrier (BBB) and act directly on the brain to alter cognitive outcomes [17].
As with most signaling networks in the body, a significant degree of variability has been observed in exerkine signaling. Factors such as exercise duration, fed-fasting status, dietary habits, genetics, and an individual's level of physical fitness all influence the pattern and intensity of exerkine release. Furthermore, recent preliminary studies suggest that sex-specific differences may further shape exerkine activity patterns. In one investigation of the proteins expressed in the saliva before and after acute exercise, nearly one in five proteins regulated by exercise responded differently in men and women [1, 18]. Yet, the broader landscape of how exerkine signaling varies by sex remains largely unexplored. Despite these gaps, research has begun to clarify the roles of individual exerkines, including IL-6 and irisin, in mediating the effects of exercise on neurodegenerative risk and cognitive health. The following section explores the molecular mechanisms by which these exercise-induced messengers exert their influence on the brain.
Irisin is released by skeletal muscle in response to exercise and circulates throughout the body, targeting fat, bone, immune cells, and the brain. Unlike many other exercise-induced compounds, irisin has the remarkable ability to cross the blood-brain barrier, granting it direct access to the CNS. Once inside the brain, irisin triggers neuroprotective gene expression in the hippocampus, including the upregulation of brain-derived neurotrophic factor (BDNF). The neuroprotective role of irisin can be better understood when this signaling axis is disrupted. For example, an irisin-deficient mouse model impairs hippocampal synaptic plasticity, promotes early memory loss, and prevents exercise-related memory improvements [19]. Moreover, crossbreeding irisin-deficient mice with an AD mouse model results in elevated levels of amyloid-beta peptide in the brain, a hallmark of neurodegeneration. This suggests that irisin could play a role in reducing plaque accumulation associated with neurodegeneration [19]. In addition, irisin was seen to protect cultured neurons by blunting the effects of neuroinflammation [20]. Taken together, these findings suggest that the release of irisin during exercise may help guard the brain against neurodegenerative diseases, offering a molecular link between exercise and cognitive resilience.
Moreover, recent evidence suggests that biological sex may influence underlying physiology and responsiveness to irisin. This sex difference results in unique molecular adaptations to exercise, thereby shifting neurodegenerative risk. Irisin increased more in females than in males immediately after the onset of high-intensity endurance exercise, during which resistance on a cycling ergometer was increased until the participant was exhausted [21]. Interestingly, exercise intensity may be a key factor for women in regulating irisin secretion. Both young men and young women increased irisin secretion by approximately 20% during low-intensity acute endurance exercise [22]. The greater irisin release in females during high-intensity endurance training could lead to increased BDNF neuroprotection and postulated plaque clearance in women vs men. Indeed, irisin levels positively correlate with BDNF and global cognitive scores and negatively correlate with amyloid deposition [23]. Furthermore, these correlations were even stronger in women [24]. These findings are consistent with sex-based differences in irisin signaling and the previously mentioned human case studies.
Beyond irisin, interleukin-6 (IL-6) is another exerkine that may influence brain function, although its neuromodulatory role has not been studied as extensively. When muscles contract, they release IL-6 into the bloodstream, which can cross the blood-brain barrier and interact directly with the central nervous system. IL-6 is notable for its dual nature: it can act as a pro-inflammatory agent—implicated in chronic inflammation—or as an anti-inflammatory signal, depending on the physiological context and duration of its presence [25]. Recent research suggests that IL-6 may promote neuron survival and reduce oxidative stress, offering a protective boost to brain health [26]. However, sustained IL-6 levels in the brain are associated with neuroinflammation and neurodegeneration [26].
After exercise, the rise in IL-6 is transient and is accompanied by the production of anti-inflammatory cytokines [27]. While the full impact of IL-6 on neurodegenerative diseases is still being investigated, current evidence points to a promising role for this short-lived exerkine in fostering a temporary anti-inflammatory state that may help shield the brain from harm and support healthy neurological function.
There also appear to be sex differences in the regulation of IL-6 as an exerkine. Notably, following resistance training, women require approximately 4 times as long as men to reach peak IL-6 levels [28]. Additionally, studies of marathon runners show a more pronounced change in IL-6 concentration before and after exercise in men compared to women [29]. While these findings are still speculative, such sex-based differences in IL-6 timing and magnitude may contribute to variations in neurological outcomes between men and women.
Exercise Enhances Brain-Derived Neurotropic Factor Levels
A significant body of research investigating the relationship between exercise and cognition has focused on BDNF. BDNF is a neuroprotective protein released in the brain and throughout the body during exercise [30]. Once active, BDNF binds to its receptor, TrkB, in the brain, where it supports neuronal survival, promotes the growth of new brain cells (neurogenesis), and strengthens the connections between neurons (synaptogenesis) [31]. Consistent with these neuroprotective functions, elevated brain BDNF expression is associated with reduced cognitive decline in both males and females [32]. Moreover, BDNF facilitates non-amyloidogenic processing of the amyloid precursor protein (APP), thereby reducing harmful Aβ accumulation implicated in neurodegenerative processes [33]. Conversely, reduced BDNF levels have been linked to more severe memory impairment, underscoring its importance in maintaining brain health [34].
The BDNF gene contains an estrogen response element, allowing estrogen to enhance BDNF gene expression when present. Consequently, reductions in estrogen are associated with decreased BDNF expression. Notably, BDNF levels decline with age in women but not in men [34], a trend that aligns with the marked drop in estrogen levels following menopause. This reduction in estrogen-mediated BDNF activity may contribute to a greater risk of cognitive decline in postmenopausal women by diminishing BDNF-induced neuroprotection. Thus, exercise emerges as an independent mechanism to upregulate BDNF and support neuroprotection, particularly in females. Preclinical models of menopause have demonstrated that female rats with surgically induced ovarian failure exhibit reduced BDNF expression; however, voluntary treadmill running improved hippocampal BDNF levels and correlated with improved cognitive outcomes [35].
The BDNF gene contains an estrogen response element, allowing estrogen to enhance BDNF gene expression when present. Consequently, reductions in estrogen are associated with decreased BDNF expression. Notably, BDNF levels decline with age in women but not in men, a trend that aligns with the marked drop in estrogen levels following menopause. This reduction in estrogen-mediated BDNF activity may contribute to a greater risk of cognitive decline in postmenopausal women by diminishing BDNF-induced neuroprotection.
Exercise Improves Immune Response
Activation of the immune system is a necessary response to infections and injury to protect the body. However, chronic or inappropriate immune activation is a central feature of a vast cohort of modern human diseases. Inflammation in the CNS, called neuroinflammation, typifies this dichotomy. In the CNS, specialized cells like microglia and astrocytes mobilize, releasing chemical messengers and reactive oxygen species. In acute circumstances such as infection, this immune response is protective for the CNS. Protracted or inappropriate neuroinflammation is maladaptive, promotes cycles of cellular damage, and is a central feature of neurodegenerative disease [36]. Mounting evidence also links systemic inflammation throughout the body to harmful changes in the brain, highlighting chronic inflammation as a key risk factor for conditions such as AD and PD [37]. Breaking this cycle—and preventing maladaptive immune activation—may be crucial for safeguarding long-term brain health.
Exercise stands out as a potent anti-inflammatory strategy, with physical activity exerting a broad influence on the body’s immune system. Physical activity helps reduce populations of pro-inflammatory cells, boosts the numbers of immune-dampening regulatory T cells, and tempers the activity of toll-like receptors (TLRs), which are key sentinels of immune defense [1]. TLRs are proteins located on immune cells that identify molecules associated with pathogens or cellular damage. When TLRs are activated, they trigger cascades of immune molecules—cytokines and chemokines—that coordinate the body’s response.
Emerging research reveals that when TLR function goes awry, it can have profound consequences for brain health. Take TLR2, for example: this immune sensor has been implicated in both neurodegeneration and neuroinflammation [36]. Elevated levels of TLR2 in neural tissue have been found to correlate with the progression of PD, potentially by interfering with cellular recycling processes—known as autophagy—and promoting the buildup of disease-associated protein plaques [39]. Intriguingly, experiments show that removing TLR2 expression can reduce plaque accumulation and even restore motor function in preclinical models of PD [36]. Interestingly, exercise appears to modulate these immune pathways. Long-term treadmill running has been shown to inhibit TLR2-driven neuroinflammation, suggesting that physical activity may help counteract some of the harmful effects associated with dysregulated TLR signaling [40].
Notably, studies indicate that men exhibit a more pronounced TLR2 response upon stimulation, suggesting possible sex-based differences in how the immune system interacts with neurological disease [41]. The observation that men exhibit a stronger TLR2 response may help explain why PD is more prevalent among males, as seen in population studies. This finding also suggests that men could gain particular benefit from long-term aerobic exercise, which has been shown to dampen TLR2-mediated neuroinflammation. However, the full impact of these sex-based differences remains to be explored. Future research is needed to determine whether the distinct TLR2 responses to exercise in men and women translate into meaningful changes in immune function and neurological outcomes.
Exercise Reduces Pathogenic Plaque Formation
Neurodegenerative diseases are often marked by the buildup of harmful protein aggregates in the brain. These aggregates are primarily composed of intrinsically disordered proteins (IDPs), molecules with flexible, unstructured regions that make them highly sensitive to changes in their cellular environment. Among the most notorious of these proteins are β-amyloid, Tau, and α-synuclein, each implicated in the pathology of conditions like AD and PD. The unstructured segments within IDPs exhibit high sensitivity to alterations in the cellular microenvironment. When the delicate balance of the brain’s microenvironment is disrupted, these proteins can misfold, clump, and form fibrils, disrupting normal cellular function [42].
In AD, for instance, a leading hypothesis centers on the extracellular accumulation of the β-amyloid 1-42 peptide (Aβ) and the formation of neurofibrillary tangles composed of Tau protein within neurons. Moreover, PD has been linked to the aggregation of both α-synuclein and Tau proteins [43]. As previously noted, research has shown that exercise can help reduce the formation of these harmful plaques, offering a promising avenue for intervention [44]. Additionally, biological sex may influence how the brain responds to exercise-induced changes in these protein aggregates, potentially affecting an individual’s risk for neurodegenerative disease. Studies led by Elisa Chelucci and colleagues have documented distinct sex-based responses to exercise in this context, underscoring the importance of personalized approaches to brain health [45].
Chelucci and colleagues conducted an observational study involving 120 healthy individuals, 60 men and 60 women. Participants were classified as either physically active or sedentary. The physically active group engaged in at least 150 minutes per week of moderate aerobic exercise over 10 years. All subjects were age-matched, with a mean age of approximately 42 years; all female participants were premenopausal. There were no significant differences in BMI among the groups, and none of the participants had a family history of AD or carried known genetic variants associated with increased AD risk. Additionally, all participants adhered to the Mediterranean diet and abstained from antioxidant supplementation. Whole blood samples were collected at least 2 days after the last exercise session and evaluated for neurodegenerative-related proteins and antioxidant responses.
Notably, women exhibited higher overall Aβ levels than men (12.69±8.32 vs 9.84±6.85 ng/mg protein). However, physical activity reduced Aβ concentrations in women to levels comparable to those observed in men (8.94±4.33 ng/mg protein in physically active women). Physical activity did not affect Aβ burden in men.
The researchers also measured miR-195 expression. miRNAs are small RNA molecules that help regulate gene activity. In this study, miR-195 was examined, as prior research has demonstrated that it regulates the BACE1 gene, an enzyme responsible for cleaving the amyloid precursor protein (APP) to generate Aβ [46]. Therefore, increased miR-195 levels can reduce Aβ production. While increased miR-195 can reduce Aβ production, only physically active women showed a corresponding decrease in Aβ levels. This suggests that women may benefit from additional miR-195-independent mechanisms to lower Aβ, or that men may be less responsive to the miR-195/BACE1 pathway. These findings highlight how exercise can have sex-specific effects on neurodegenerative risk, underscoring the importance of personalized approaches to brain health.
Chelucci and colleagues next turned their attention to Tau. Interestingly, men and women did not differ in their overall Tau levels (7.85±7.42 vs 8.09±7.65 ng/mg protein, respectively). However, when participants were grouped by physical activity, those who exercised regularly had significantly lower Tau concentrations than their sedentary counterparts (5.61±3.54 vs 9.48±8.89 ng/mg protein). A closer look revealed that active men experienced a marked reduction in Tau compared to sedentary men (5.61±4.25 vs.9.57±8.82 ng/mg protein). Active women also tended to have lower Tau levels than sedentary women (5.62±2.50 vs. 9.41±9.0mg/mg protein). However, this trend did not reach statistical significance, possibly due to the study’s limited sample size.
Despite these nuances, the overarching finding was clear: long-term, consistent physical activity was associated with reduced Tau, a change linked to decreased risk for tauopathies, such as AD and PD. The study also uncovered an intriguing connection between Tau and testosterone. Across both sexes, physically active individuals had higher testosterone levels, and Tau concentrations were inversely correlated with testosterone. Active men’s testosterone was 673.01±221.38 pg/mL vs sedentary men’s 438.52±129.29 pg/mL; active women’s testosterone was 36.08±14.84 pg/mL vs sedentary women’s 24.77±15.11 pg/mL. These results suggest that exercise-induced hormonal changes may modulate Tau and, by extension, neurodegenerative risk.
Chelucci and colleagues propose that the pattern of IDP burden identified in their research aligns with the sex bias seen in neurological disease, indicating that regular physical activity may confer sex-specific cognitive benefits by influencing pathogenic IDPs. Their findings revealed that women exhibited a greater Aβ burden than men, which is consistent with the higher risk of AD observed in women; notably, physically active women demonstrated reduced Aβ levels and a lower risk of cognitive decline. However, the relationship between IDP burden and disease risk appears more complex in men. Men are at elevated risk for PD, which correlates with pathogenic α-synuclein and Tau accumulation [43]. In this cohort, α-synuclein levels were higher in women, and physical activity did not affect them. Conversely, Tau levels were decreased in active men but remained unchanged in women. This mixed response underscores the necessity for further mechanistic investigations into the interplay between α-synuclein, Tau, and biological sex differences.
Exercise Optimizes Antioxidant Response
Under normal physiological conditions, our cells constantly generate reactive oxygen species (ROS) and reactive nitrogen species (RNS) as natural byproducts of metabolism, especially during mitochondrial energy production and immune defense against pathogens. These molecules include familiar names like superoxide anion (O₂•⁻), hydroxyl radical (•OH), hydrogen peroxide (H₂O₂), nitric oxide radical (•NO), and peroxynitrite (ONOO⁻), which is the product of •NO and O₂•⁻. ROS/RNS species are not only products of these vital metabolic pathways but also play crucial roles in cell signaling, synaptic plasticity, and cellular defense mechanisms [47]. To keep these reactive molecules in check, our bodies rely on protective enzymes—such as superoxide dismutase, catalase, and glutathione peroxidase—as well as antioxidant molecules such as vitamin C, vitamin E, and glutathione. Problems arise when ROS and RNS production outpaces the cell’s buffering capacity, tipping the balance toward pathological oxidative stress. This excess can damage proteins, lipids, and DNA, setting the stage for cellular dysfunction and disease.
Oxidative stress serves as a key pathological factor in neurodegeneration. It induces direct damage to nucleic acids, proteins, and lipids. Oxidative modification of these macromolecules results in pathogenic IDP aggregation, compromised cellular membrane integrity, and DNA damage. Mitochondrial DNA damage is particularly critical, as it impairs mitochondrial enzyme function, further increasing ROS production. This unchecked ROS generation establishes a self-perpetuating cycle of oxidative damage, ultimately leading to cell loss. This is particularly harmful to neurons, as they have a high metabolic rate and the capacity to produce ROS, yet limited resources to buffer oxidizing agents [47].
Furthermore, oxidative stress–induced neurotoxicity can activate a neuroinflammatory response in glial cells, which, in turn, promotes increased ROS production [48]. Multiple strategies have been suggested to mitigate the effects of oxidative stress (see Healthspan resources), including exercise. Chelucci and colleagues chose to examine how biological sex alters the oxidative stress response to exercise.
The researchers measured Nrf2 concentration in red blood cells from physically active and sedentary men and women. Nrf2 is a transcription factor considered a master regulator of oxidative stress. Nrf2 can bind to DNA to increase the expression of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase [47], thereby reducing the ROS load in the cell. There was no difference in Nrf2 concentration between men and women overall (15.97 ± 6.27 vs. 15.85 ± 6.24; Abs ratio Nrf2/Abs total proteins, µg/µL). However, physically active men and women had higher Nrf2 levels than sedentary controls. Physically active women had 18.81 ± 6.25 µg/µL of Nrf2 versus 14.20 ± 5.67 µg/µL in sedentary women; physically active men had 19.72 ± 5.54 µg/µL of Nrf2 versus 13.10 ± 5.24 µg/µL in sedentary men. This indicates that long-term engagement in consistent exercise increases the capacity to blunt pathogenic oxidative stress, lowering neurodegenerative risk.
The authors further investigated Nrf2 signaling by measuring levels of histone deacetylase 6 (HDAC6) and miR-153. HDAC6 was examined because inhibition of HDAC6 has been shown to increase Nrf2 activity; lowering HDAC6 levels would therefore buffer oxidative stress by promoting Nrf2-induced gene protection [49]. HDAC6 levels did not differ by sex. Physically active individuals, however, showed reduced HDAC6 expression compared with sedentary individuals, thereby enhancing Nrf2-driven protection. Nrf2 is also regulated by miR-153, a small RNA molecule that can target proteins limiting Nrf2 activation of the antioxidant program [50]. Lower levels of miR-153 are associated with greater protection from oxidative stress via Nrf2. In this study, miR-153 was reduced only in physically active males, suggesting a sex-specific mechanism. These findings indicate that exercise enhances Nrf2-driven antioxidant defenses in both sexes, though underlying mechanisms may differ—offering intriguing possibilities for treating neurodegenerative diseases through sex-specific Nrf2 pathways.
To further examine how exercise influences the body’s antioxidant defenses, Chelucci and colleagues exposed participants’ plasma samples to a battery of reactive species—peroxyl radicals, hydroxyl radicals, and peroxynitrite. This experiment measured each individual’s capacity to neutralize these damaging molecules. Interestingly, overall antioxidant capacity did not differ significantly between men and women. However, when the data were analyzed by physical activity level, striking sex differences emerged. Athletic men demonstrated a greater ability to buffer all three types of radicals compared to their sedentary counterparts.
In contrast, physically active women showed improved antioxidant capacity only against peroxynitrite radicals; their ability to neutralize peroxyl and hydroxyl radicals did not increase with exercise. These findings suggest that biological sex sets distinct limits on how exercise can enhance antioxidant defenses. Given the role of reactive oxygen and nitrogen species in neurodegenerative disease, this may mean that women are more vulnerable to oxidative damage from certain radicals, despite the benefits of physical activity.
Tailoring Exercise to the Brain: Lessons from Sex Differences in Neuroprotection
Exercise is a powerful modulator of systems physiology, influencing nearly every mechanism implicated in neurodegenerative disease—from oxidative stress and neuroinflammation to synaptic plasticity and protein clearance. Biological sex further shapes these pathways, determining how the brain responds to physical activity and how effectively exercise may offset neurodegenerative risk.
Research shows that while both men and women benefit from regular exercise, the physiological and molecular adaptations differ between the sexes. Women tend to experience greater cognitive improvements and reductions in amyloid burden, while men may exhibit stronger responses in Tau reduction, antioxidant capacity, and hormone-linked neuroprotection.
Women tend to show greater improvements in cognition and reductions in amyloid burden following aerobic activity, particularly after menopause. High-intensity endurance exercise appears to increase irisin levels, which stimulate brain-derived neurotrophic factor (BDNF) and enhance hippocampal resilience. However, women’s antioxidant gains are often more selective, highlighting the importance of maintaining aerobic consistency.
Men, on the other hand, may see stronger effects on Tau reduction, antioxidant capacity, and testosterone-related neuroprotection. Both resistance and aerobic training help modulate immune signaling pathways (such as TLR2), offering anti-inflammatory benefits that can stabilize neuronal health.
Understanding these sex-based differences matters because it opens the door to more personalized approaches to brain health.
Below are key takeaways summarizing observed sex-based differences in the neurological benefits of exercise, along with an overview of how various exercise components may support cognitive function in each group.
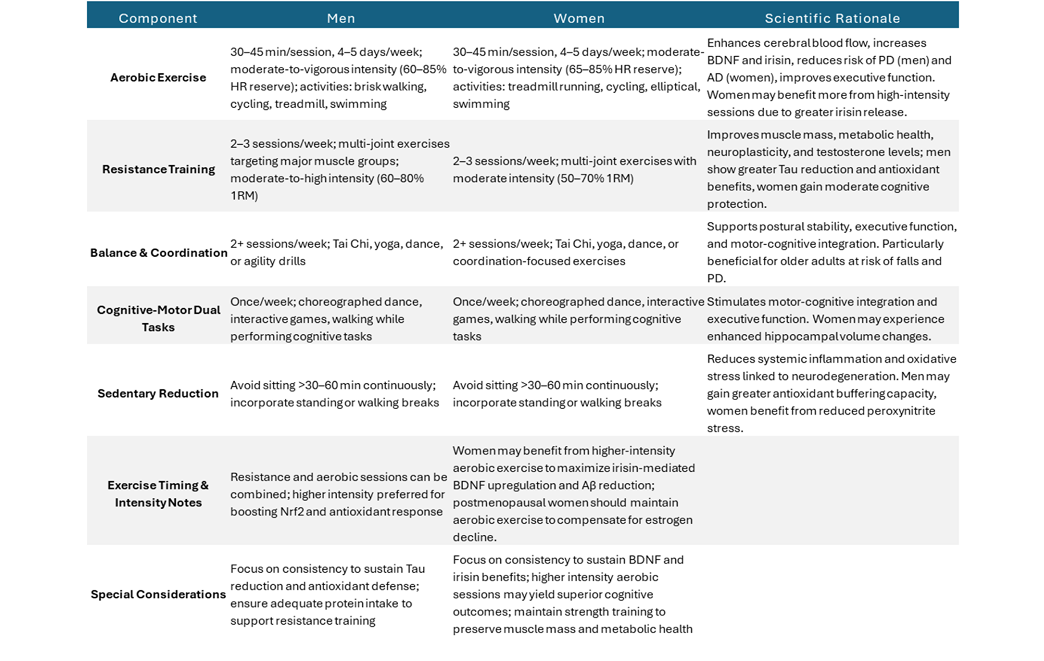
Table 3: Exercise recommendations for cognitive health in men and women
Together, these findings highlight that while exercise remains universally beneficial, its optimal type, intensity, and physiological impact can vary between men and women. The interpretations provided here reflect the current state of available research and may evolve as new studies clarify underlying mechanisms and long-term outcomes. Readers are encouraged to view these insights as a framework for understanding how movement supports brain resilience—not as prescriptive medical advice.
Ultimately, consistent engagement in both aerobic and resistance exercise remains one of the most evidence-supported ways to protect the brain, regardless of sex. As science advances, personalized exercise approaches informed by biology and life stage may help maximize these neuroprotective effects and extend cognitive vitality across the lifespan.
- Collaborators, G.B.D.N.S.D., Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol, 2024. 23(4): p. 344–381.
- Wang, S., et al., The Expanding Burden of Neurodegenerative Diseases: An Unmet Medical and Social Need. Aging Dis, 2024. 16(5): p. 2937–2952.
- Potokiri, A. and H. Wang, Proteostasis and Neuroinflammation in Alzheimer's Disease. J Integr Neurosci, 2025. 24(9): p. 39826.
- Abdol Samat, H.N., et al., The Interplay of Inflammation and Gut-Microbiota Dysbiosis in Alzheimer's Disease: Mechanisms and Therapeutic Potential. Int J Mol Sci, 2025. 26(18).
- Steffan, D., et al., Mitochondrial Aging in the CNS: Unravelling Implications for Neurological Health and Disease. Biomolecules, 2025. 15(9).
- Kuo, C.J., et al., Transcriptional Dysregulation of Autophagy in Aging and Potential Interventions: Insights Into TFEB and FOXOs. Front Biosci (Landmark Ed), 2025. 30(9): p. 38730.
- Li, W.Q., et al., Exercise as a multitarget therapy: modulating myokines, neurotrophins, and inflammation in Parkinson's disease. Front Aging Neurosci, 2025. 17: p. 1580029.
- Su, Y. and Z. Su, Effects of exercise on neuroinflammation in age-related neurodegenerative disorders. Eur J Med Res, 2025. 30(1): p. 909.
- Tari, A.R., et al., Neuroprotective mechanisms of exercise and the importance of fitness for healthy brain ageing. Lancet, 2025. 405(10484): p. 1093–1118.
- Santiago, J.A. and J.A. Potashkin, Physical activity and lifestyle modifications in the treatment of neurodegenerative diseases. Front Aging Neurosci, 2023. 15: p. 1185671.
- Cortes, C.J. and Z. De Miguel, Precision Exercise Medicine: Sex Specific Differences in Immune and CNS Responses to Physical Activity. Brain Plast, 2022. 8(1): p. 65–77.
- Baker, L.D., et al., Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol, 2010. 67(1): p. 71–9.
- Sofi, F., et al., Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med, 2011. 269(1): p. 107–17.
- Barha, C.K., et al., Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front Neuroendocrinol, 2017. 46: p. 71–85.
- Varma, V.R., et al., Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus, 2015. 25(5): p. 605–15.
- Chow, L.S., et al., Exerkines in health, resilience and disease. Nat Rev Endocrinol, 2022. 18(5): p. 273–289.
- Kukla-Bartoszek, M. and K. Glombik, Train and Reprogram Your Brain: Effects of Physical Exercise at Different Stages of Life on Brain Functions Saved in Epigenetic Modifications. Int J Mol Sci, 2024. 25(22).
- Franco-Martinez, L., et al., Differences on salivary proteome at rest and in response to an acute exercise in men and women: A pilot study. J Proteomics, 2020. 214: p. 103629.
- Islam, M.R., et al., Exercise hormone irisin is a critical regulator of cognitive function. Nat Metab, 2021. 3(8): p. 1058–1070.
- Wang, K., et al., Irisin Exerts Neuroprotective Effects on Cultured Neurons by Regulating Astrocytes. Mediators Inflamm, 2018. 2018: p. 9070341.
- Zugel, M., et al., The role of sex, adiposity, and gonadectomy in the regulation of irisin secretion. Endocrine, 2016. 54(1): p. 101–110.
- Kraemer, R.R., et al., A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm Metab Res, 2014. 46(2): p. 150–4.
- Lourenco, M.V., et al., Cerebrospinal fluid irisin correlates with amyloid-beta, BDNF, and cognition in Alzheimer's disease. Alzheimers Dement (Amst), 2020. 12(1): p. e12034.
- Dicarlo, M., et al., Irisin Levels in Cerebrospinal Fluid Correlate with Biomarkers and Clinical Dementia Scores in Alzheimer Disease. Ann Neurol, 2024. 96(1): p. 61–73.
- Fuster, J.J. and K. Walsh, The good, the bad, and the ugly of interleukin-6 signaling. EMBO J, 2014. 33(13): p. 1425–7.
- Kummer, K.K., et al., Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine, 2021. 144: p. 155582.
- Brandt, C. and B.K. Pedersen, The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol, 2010. 2010: p. 520258.
- Benini, R., et al., Influence of sex on cytokines, heat shock protein and oxidative stress markers in response to an acute total body resistance exercise protocol. J Exerc Sci Fit, 2015. 13(1): p. 1–7.
- Abbasi, A., et al., Sex-specific variation in signaling pathways and gene expression patterns in human leukocytes in response to endotoxin and exercise. J Neuroinflammation, 2016. 13(1): p. 289.
- Rasmussen, P., et al., Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol, 2009. 94(10): p. 1062–9.
- Bagit, A., G.C. Hayward, and R.E.K. MacPherson, Exercise and estrogen: common pathways in Alzheimer's disease pathology. Am J Physiol Endocrinol Metab, 2021. 321(1): p. E164–E168.
- Buchman, A.S., et al., Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology, 2016. 86(8): p. 735–41.
- Nigam, S.M., et al., Exercise and BDNF reduce Abeta production by enhancing alpha-secretase processing of APP. J Neurochem, 2017. 142(2): p. 286–296.
- Barros-Aragao, F.G.Q., et al., Physical activity in Alzheimer's disease prevention: Sex differences and the roles of BDNF and irisin. Front Neuroendocrinol, 2025. 77: p. 101189.
- Rashidy-Pour, A., et al., Voluntary exercise and estradiol reverse ovariectomy-induced spatial learning and memory deficits and reduction in hippocampal brain-derived neurotrophic factor in rats. Pharmacol Biochem Behav, 2019. 187: p. 172819.
- Adamu, A., et al., The role of neuroinflammation in neurodegenerative diseases: current understanding and future therapeutic targets. Front Aging Neurosci, 2024. 16: p. 1347987.
- Perry, V.H., T.A. Newman, and C. Cunningham, The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci, 2003. 4(2): p. 103–12.
- Popotas, A., et al., Sex-related immunity: could Toll-like receptors be the answer in acute inflammatory response? Front Immunol, 2024. 15: p. 1379754.
- Gorecki, A.M., C.C. Anyaegbu, and R.S. Anderton, TLR2 and TLR4 in Parkinson's disease pathogenesis: the environment takes a toll on the gut. Transl Neurodegener, 2021. 10(1): p. 47.
- Koo, J.H., et al., Treadmill exercise produces neuroprotective effects in a murine model of Parkinson's disease by regulating the TLR2/MyD88/NF-kappaB signaling pathway. Neuroscience, 2017. 356: p. 102–113.
- Lefevre, N., et al., The Number of X Chromosomes Influences Inflammatory Cytokine Production Following Toll-Like Receptor Stimulation. Front Immunol, 2019. 10: p. 1052.\
- Tsoi, P.S., et al., Aggregation of Disordered Proteins Associated with Neurodegeneration. Int J Mol Sci, 2023. 24(4).
- Zhang, X., et al., Tau Pathology in Parkinson's Disease. Front Neurol, 2018. 9: p. 809.
- Piccarducci, R., et al., Apolipoprotein E Polymorphism and Oxidative Stress in Human Peripheral Blood Cells: Can Physical Activity Reactivate the Proteasome System through Epigenetic Mechanisms? Oxid Med Cell Longev, 2021. 2021: p. 8869849.
- Chelucci, E., et al., Sex Differences in Blood Accumulation of Neurodegenerative-Related Proteins and Antioxidant Responses to Regular Physical Exercise. J Mol Neurosci, 2024. 74(4): p. 105.
- Zhu, H.C., et al., MicroRNA-195 downregulates Alzheimer's disease amyloid-beta production by targeting BACE1. Brain Res Bull, 2012. 88(6): p. 596–601.
- Serban, M., C. Toader, and R.A. Covache-Busuioc, The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration. Int J Mol Sci, 2025. 26(15).
- Wang, X., et al., Unraveling the Vicious Cycle: Oxidative Stress and Neurotoxicity in Neurodegenerative Diseases. FASEB Bioadv, 2025. 7(8): p. e70041.
- Wang, B., et al., Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med, 2012. 52(5): p. 928–36.
- Qu, Y., et al., MiR-153 Prevents NRF2 Nuclear Translocation to Drive Hypoperfusion-Related Cognitive Deficits by Targeting KPNA5. J Neurosci, 2025. 45(38).
Related studies