Synergistic Effects of Rapamycin and Exercise for Maximizing Muscular Strength
Strength, not just size, predicts healthy aging. While bigger muscles help, it’s strength that best predicts independence and survival in older adults. Something as simple as grip strength has been shown to forecast disability, hospitalization, and even lifespan.
The nervous system is key to early strength gains. When people start resistance training, strength improves almost immediately—not because muscles have grown yet, but because the brain and nerves learn to recruit muscle fibers more efficiently. Hypertrophy takes 10–12 weeks, but neural gains show up in the first few sessions.
Strength training remains effective at any age. Even in older adults with little or no training history, meaningful strength gains are achievable. Neural adaptations make strength improvements possible even when muscle growth is slower or harder to achieve.
mTORC1 overactivation drives decline with age. In aged muscle, mTORC1 becomes chronically “stuck on,” suppressing autophagy, promoting senescence, and impairing muscle responsiveness to exercise. Rapamycin’s value lies in resetting this balance.
Rapamycin’s effects depend on the dose. High, frequent doses can blunt muscle growth, but small, intermittent doses (3–6 mg weekly in humans) appear to support recovery and repair. The difference is like tapping the brakes to regain control versus slamming them and stopping the car altogether.
Animal studies show rapamycin preserves aging muscle. In aged rats, six weeks of low-dose rapamycin boosted tibialis anterior muscle mass by 10–15%, cut the number of weak, atrophic fibers in half, and reduced signs of muscle degeneration by ~50%. High doses, in contrast, showed no benefit.
Resistance exercise and rapamycin may lower the “dose” of each other needed. By pairing rapamycin with moderate resistance exercise, older adults may achieve strength and recovery benefits with less total drug exposure and lower exercise volume — a particularly important advantage for those with limited tolerance. A new clinical trial will test whether once-weekly rapamycin plus resistance training improves strength and endurance in older adults.
Overview
Rapamycin has become one of the most intriguing drugs in aging research, best known for its ability to extend lifespan in multiple species. Yet when it comes to muscles, its role is less clear. At first glance, rapamycin seems like it might even be harmful. After all, it works by suppressing mTORC1, a cellular pathway that acts as a master switch for cell growth. This is the same pathway that weightlifters, athletes, and even everyday exercisers rely on to build and repair muscle after training.
In young, healthy tissue, mTORC1 is indispensable. It senses nutrients, growth factors, and mechanical stress from exercise, then triggers muscle fibers to repair and grow stronger. Without it, adaptation to training would grind to a halt. But with aging, these dynamics change. Scientists have found that mTOR activity often remains chronically elevated with age, no longer switching cleanly between growth and rest. It is a bit like a car with the accelerator pedal stuck down—always pressing forward, never easing off. What once powered healthy growth now overshoots the mark, driving the buildup of unhealthy tissue, fueling inflammation, and suppressing the cell’s cleanup systems, such as autophagy. In other words, what was once a force for healthy tissue growth and renewal can become a contributor to decline.
That apparent contradiction has sparked debate: if rapamycin slows growth signals, could it also blunt the very adaptations we need for strength? The answer, emerging from both basic science and early human protocols, seems to lie in how the drug is used.
Dosing approaches are key in eliciting desired effects. Chronic, high-frequency rapamycin intake may impair hypertrophy and muscle recovery. Conversely, cyclic low-dose rapamycin intake, especially when paired with resistance or moderate-intensity exercise, may assist in countering mTORC1 overactivation that develops with age or in disease states such as sarcopenia (age-related loss of muscle mass). This, in turn, could promote autophagy, optimize muscle repair, and improve skeletal muscle metabolic function.
This matters because muscle strength is more than just a gym metric. One of the less discussed but critical areas of extending healthspan is preservation of muscular strength, the maximum amount of force that a muscle or muscle group can produce [1]. While hypertrophy (increase in muscle fiber size) is important, muscular strength is especially predictive of quality of life with age and in disease states. While much of the rapamycin literature has discussed its benefits for anti-aging and skeletal muscle health by shifting the balance toward cellular repair and clearance, rather than constant growth signaling, this leaves much to be explored on the frontier of its effects on muscular strength.
Now, a new line of investigation is beginning to explore exactly that. Based on available data, rapamycin and resistance training may have synergistic benefits. Dr. Bradley Stanfield et al. 2024 [2] have developed the first proposed clinical trial protocol for evaluating the effects of intermittent rapamycin dosing on muscular strength and overall health.
In this article, we will dive into the specific importance of muscular strength, and how it differs from hypertrophy, as well as explore the benefits of rapamycin for skeletal muscle health both alone, and when combined with resistance training.
Why Focus on Muscular Strength?
In research and athletic settings, muscular strength is usually measured by the heaviest load a person can lift once—the classic “one-repetition maximum.” But strength is more than a laboratory test. With advancing age, the stakes become clear: sarcopenia (age-related muscle loss), falls, fractures, and osteoporosis all become far more common [3,4,5]. For decades, researchers have focused on preserving muscle mass as a bulwark against these risks. Yet size alone is not the whole story.
Consider the simple act of catching yourself when you trip. Success depends not only on having enough muscle, but also on how quickly and efficiently those muscles respond. Neurological decline with age—slowed reflexes, impaired proprioception (the body’s sense of position in space), and weaker communication between nerves and muscles—plays just as large a role as loss of mass. [3] That’s why strength and power, the ability to generate force rapidly, are more functionally meaningful than muscle size alone when it comes to preserving independence and quality of life. To this point, aging individuals who maintain muscular strength exhibit lower rates of mortality, frailty, and disability, and report a greater capacity to perform activities of daily living (ADLs) [6].
While hypertrophy, or increased muscle fiber size, is positively associated with muscular strength, a primary driver of strength development is neurological [8]. At its core, strength is not just about the bulk of the muscle but about the efficiency of the “wiring” that connects brain to muscle. Muscular strength is largely a function of the communication strength and efficiency between the central nervous system, and skeletal muscle via peripheral motor neurons [1, 7]. To think about this in practice, we must understand how our CNS and muscles are connected. Each of our muscle fibers are supplied or innervated by nerves that carry instructions from our brain or spinal cord out to our periphery. The specific type of specialized nerve cell that connects the end of a peripheral nerve to a muscle fiber or group of fibers is called a motor neuron, while a single motor neuron and all of the muscle fibers it innervates are called a motor unit. When performing any basic day-to-day activity, such as standing up from a chair, our brain sends instructions in the form of electrical signals that are transmitted down our spinal cord, out through our peripheral nerves, and into these motor units, which ultimately allow muscle contraction to happen.
The amount of force we generate (strength) and the speed at which we generate it (power) are governed entirely by our ability to stimulate these motor units as efficiently as possible. Stronger individuals are better able to recruit more motor units at lower intensities, activate high-threshold motor units sooner, and fire these units more synchronously and with greater frequency [1, 8]. This helps explain why impressive strength is not always accompanied by extreme muscle mass. Think of it like an orchestra: hypertrophy adds more instruments, but strength comes from the conductor ensuring they all play in harmony at the right time.
You may notice that after starting a resistance exercise program, you have the capacity to increase the weight lifted and number of repetitions performed per set on most exercises, but don’t see meaningful changes in muscle size for quite some time. That’s because neurological adaptations, which enhance our ability to recruit and coordinate motor units to generate force, begin almost immediately and can continue improving for years. In contrast, noticeable hypertrophy typically requires a minimum of 10–12 weeks of consistent training to emerge [1].
...neurological adaptations, which enhance our ability to recruit and coordinate motor units to generate force, begin almost immediately and can continue improving for years. In contrast, noticeable hypertrophy typically requires a minimum of 10–12 weeks of consistent training to emerge
Muscular Strength is Critically Important for Healthy Aging
For older adults, the distinction between muscle size and muscle strength becomes especially important. Resistance training to improve strength can help offset neurological decline and impaired motor control, which contribute significantly to age-related falls and functional limitations [9, 10]. While cardiovascular fitness promotes longevity via improving vascular health, reducing cardiovascular workload, improving mitochondrial function, and reducing systemic inflammation [11, 12], muscular strength is essential for mobility, balance, and injury prevention.
Age-related declines in strength, referred to as dynapenia, are a major contributor to frailty [13]. What makes dynapenia so insidious is that it predicts disability, hospitalization, and even mortality independently of muscle mass [13]. The ability of a muscle to generate adequate force to meet demands imposed by ADLs and unexpected circumstances such as falls or loss of balance, is critical to maintain functional independence with age. In fact, grip strength is one of the most powerful clinical predictors of overall health and lifespan in aging adults [14]. While hypertrophy becomes more difficult, but still achievable, with age [15], muscular strength remains highly attainable, even with minimal training history [16, 17]. Due to the natural occurrence of muscle atrophy, muscle fiber loss, reduced satellite cell activity, and development of anabolic resistance with age, maximizing neural adaptations that optimize motor unit recruitment is especially important [15, 18]. This makes strength training a valuable strategy for extending healthspan.
Applications of Rapamycin for Maintenance of Muscular Strength
Rapamycin has emerged as a promising, low-risk intervention for enhancing muscle recovery, preserving strength, and potentially supporting hypertrophy. Briefly, rapamycin exerts its effects primarily by inhibiting the mTORC1 pathway [19,20,21]. In healthy skeletal muscle, this pathway is indispensable: it drives anabolic signaling required for muscle growth and the preservation of muscle mass [19], underscoring early concerns that rapamycin intake would over-suppress this anabolic pathway, with high doses or high-frequency intake shown to impair muscle hypertrophy through downregulation of anabolic signaling and upregulation of autophagy [22].
However, it is now understood that rapamycin’s mechanism of action varies based on dose and dosing frequency. Emerging evidence suggests that blocking mTORC1 activity can be beneficial in situations where its function is dysregulated and chronically overactive, such as in aging, cancer, diabetes, sarcopenia, or normal aging where overactive mTORC1 signaling has been implicated in cellular damage and degeneration [23,24,25,26]. Specifically, overactivation of mTORC1 impairs autophagy, promoting the accumulation of damaged proteins and organelles, and driving cellular senescence. When mTORC1 signaling is locked in the “on” position, this essential repair mechanism is suppressed, allowing molecular debris to build up inside the cell [24, 27].The result is the acceleration of cellular senescence—cells that are metabolically active but dysfunctional, secreting inflammatory molecules and resisting removal. As senescent cells accumulate, they undermine tissue health and resilience, making muscles less responsive to the normal benefits of exercise. This helps explain why sarcopenia, anabolic resistance, and age-related deterioration of muscle morphology are increasingly linked to the unchecked activity of mTORC1 [24, 27].
As senescent cells accumulate, they undermine tissue health and resilience, making muscles less responsive to the normal benefits of exercise. This helps explain why sarcopenia, anabolic resistance, and age-related deterioration of muscle morphology are increasingly linked to the unchecked activity of mTORC1.
In this context, carefully titrated rapamycin dosing can help restore equilibrium, dialing down excessive growth signaling without completely silencing mTORC1. By doing so, rapamycin may shift muscle biology toward repair and clearance, supporting autophagy, reducing senescence, and improving mitochondrial function and metabolic health [24, 26, 27]. Evidence suggests that low-to-moderate weekly dosing (for example, 3–6 mg) may enhance muscle recovery and performance [24, 26, 27] and may be particularly impactful in middle-aged and older adults, where dysregulated mTORC1 signaling is most pronounced.
The key is dosage. Low-dose rapamycin works like tapping the brakes—slowing excessive signaling without stopping the car. High doses, by contrast, can slam the brakes too hard, halting not just harmful overactivation but also the beneficial anabolic activity needed for healthy adaptation.
It is important to recognize that mTORC1 is not the only signaling pathway responsible for building and maintaining muscle. Muscle protein synthesis is a complex, redundant process governed by multiple pathways that can operate independently of mTORC1 [28, 29], making it inappropriate to conflate mTORC1 inhibition with a complete attenuation of skeletal muscle hypertrophy. This redundancy is not accidental—it is an evolutionary safeguard. Because muscle function is essential for survival, the body relies on a network of overlapping mechanisms rather than a single molecular switch. As a result, inhibiting mTORC1 does not mean that muscles lose the ability to grow or adapt altogether. Instead, other pathways can step in to maintain protein synthesis and repair, especially in the presence of exercise stimuli.
This perspective has begun to shape how researchers interpret rapamycin’s role in skeletal muscle. In an editorial titled “Rapamycin protects aging muscle,” Tang et al. [30] argued that rapamycin should not be viewed as a blunt tool that shuts down growth. Instead, they provided a framework for understanding rapamycin's protective effects on skeletal muscle, noting that overactivity of mTORC1 was associated with muscular damage and degeneration in aging cells, and that mTORC1 inhibition was helpful for preserving muscle mass and healthy cell growth. From this vantage point, rapamycin is less a growth inhibitor and more a modulator—restoring balance in an aging system where the scales have tipped too far toward unchecked growth signaling.
Experimental evidence supports this framework. This pattern was illustrated in a study by Joseph et al. [24], discussed in a prior review [31], which used an aged animal model to examine how mTOR inhibitor dosing affects sarcopenia and anabolic resistance. In this study [24], 24-month-old rats were assigned to receive either a low-dose mTOR inhibitor, a high-dose mTOR inhibitor, or no mTOR inhibition (control) for six weeks. Their muscle quality and fiber size were compared to a younger reference group (nine months old). As expected, anabolic resistance and impaired muscle quality were observed in aged rat tissue. However, low-dose mTOR inhibition increased muscle cross-sectional area, preserved fast-twitch fiber density, and slowed the age-related decline in fiber size, whereas high-dose mTOR inhibition offered no such benefit. In fact, rats given the low dose displayed a 10–15% mean increase in mass of the tibialis anterior muscle, a 50% reduction in small, atrophic fibers characteristic of aged muscle, and an approximately 50% decrease in central nuclei, a marker of muscle degeneration [24]. By contrast, the high dose conferred no such benefit.
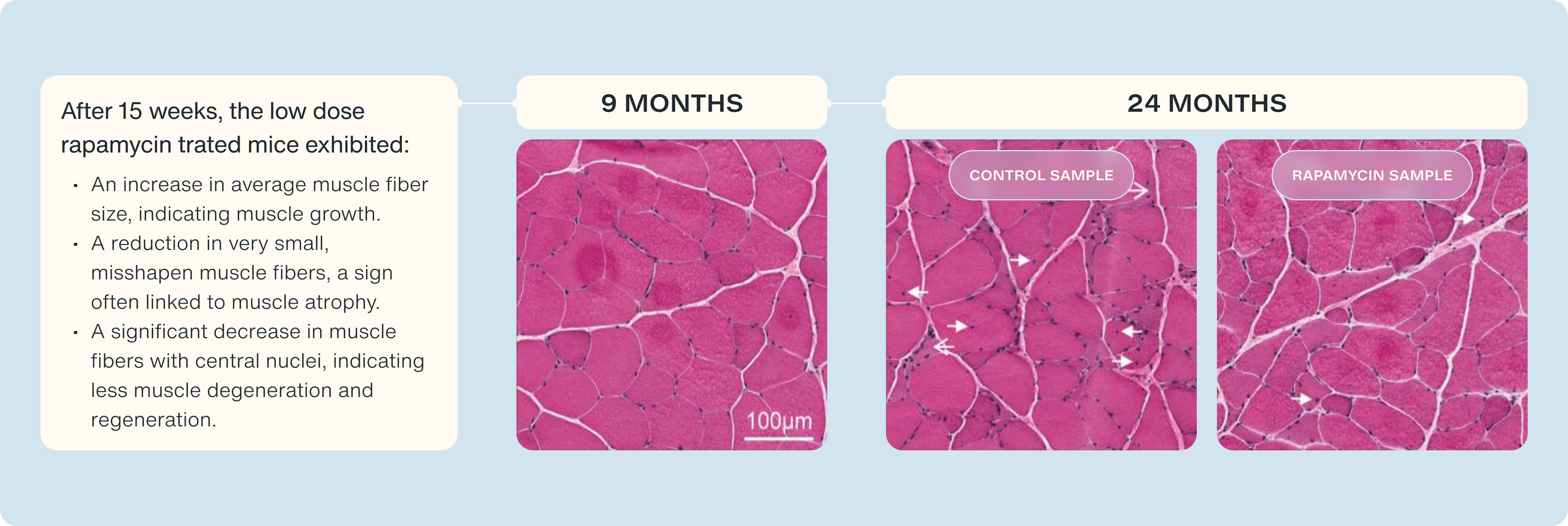
These findings suggest that appropriately titrated rapamycin dosing may strike a balance between avoiding excessive mTORC1 inhibition and supporting muscle recovery, muscle fiber quality, and anabolic signaling. As muscle mass and anabolic sensitivity decline steadily with age [15], preserving muscle function is critical for extending healthspan. Rapamycin’s dual role—enhancing autophagy and reducing muscular degeneration—provides a supported rationale for its use as a muscle-preserving intervention across the lifespan.
To date, no human studies have directly tested the combined effects of rapamycin and resistance training on muscular strength. However, tightly controlled animal studies [23, 24, 32] and early-phase human trials [28, 30] suggest that rapamycin can help preserve muscle mass in aging and disease.
A particularly promising development is the randomized controlled trial outlined by Stanfield et al. [2], which aims to examine the effects of once-weekly rapamycin (6 mg) in older adults (65–85 years) undergoing a structured, thrice-weekly resistance training program over 13 weeks. The trial is designed to assess muscular strength, muscular endurance, and systemic inflammation at both baseline and post-intervention. While data collection is still in progress (Australia New Zealand Clinical Trial Registry: ACTRN12624000790549), the study offers a rare and timely opportunity to explore how targeted mTOR modulation might interact with resistance training in an aging population.
If the anticipated outcomes are realized, this research could deliver the first human evidence that rapamycin enhances the adaptive response to resistance exercise, potentially lowering the volume or intensity of training required to maintain or improve muscular strength. This would have significant implications for older adults, particularly those with cardiometabolic disease or limited exercise tolerance, by expanding access to strength-preserving interventions that were once reserved for those capable of sustaining higher-intensity programs.
Resistance training is already well-established in the literature as a means to improve strength, neuromuscular function, proprioception, bone mineral density, and reduce fall risk [9, 10]. However, many older adults, particularly those with cardiometabolic conditions, struggle to engage in or adhere to high-intensity resistance training [33, 34]. If long-term intake of rapamycin can enhance muscle maintenance, recovery, or adaptability, it is plausible that lower doses or volumes of resistance exercise may be sufficient to maintain muscular strength in aging populations. This could increase the accessibility of strength-promoting interventions for older adults, reduce training burden, and improve long-term adherence.
The American College of Sports Medicine (ACSM) recommends that adults train each major muscle group 2–3 days per week, performing 2–4 sets of 8–12 repetitions at 60–80% of their one-repetition maximum, with 2–3 minutes of rest between sets, to optimize musculoskeletal and general health [35]. While these guidelines remain the gold standard, for individuals with age-related limitations or chronic disease, physical activity is a crucial longevity habit even in smaller, less frequent doses. Rapamycin may enable meaningful benefits even when training volume or intensity falls short of the optimal range. Figure 1 illustrates a potential framework for the synergistic benefits of combining resistance training with rapamycin dosing.
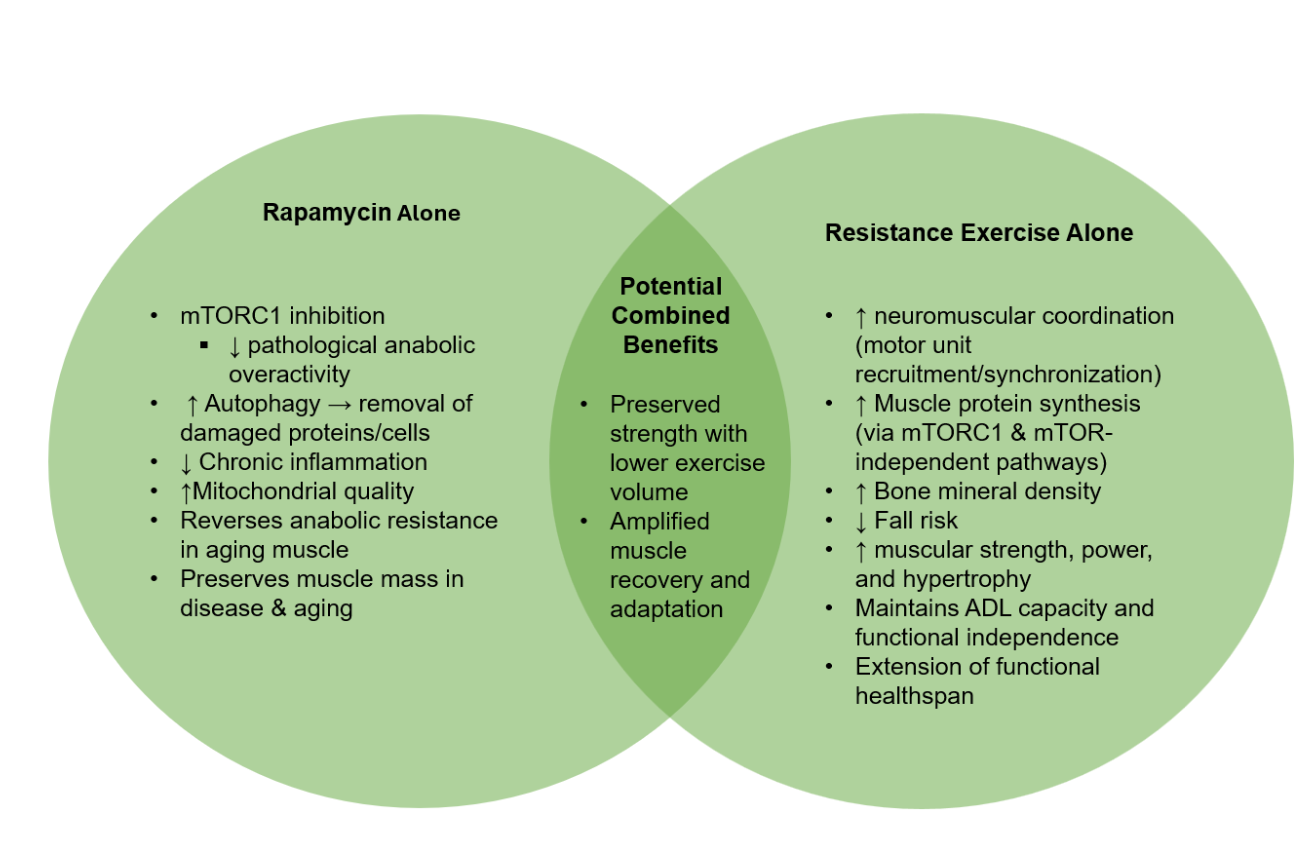
Figure 1. A framework for the synergistic benefits of concomitant rapamycin dosing and chronic resistance exercise on muscular strength and hypertrophy.
Conclusion
Muscular strength is essential for healthy aging, underpinning mobility, independence, and quality of life. While resistance exercise remains the gold standard for developing strength, emerging evidence suggests that rapamycin may serve as a physiological “primer,” tuning muscle biology so that the same exercise effort yields greater returns. If validated, this dual approach could redefine how we preserve musculoskeletal health, particularly in aging and diseased populations.
The hypothesized synergy between rapamycin and resistance training represents a promising frontier in extending healthspan and functional capacity. In practical terms, it is like having two complementary levers: exercise provides the external mechanical stimulus to build strength, while rapamycin modulates the internal cellular environment—reducing excessive growth signaling, clearing damaged components, and enhancing recovery. When pulled together, these levers may amplify each other’s effects, allowing smaller, more achievable investments of exercise to produce disproportionately greater benefits.
Ongoing trials will be critical in determining the magnitude of these synergistic benefits. Until then, the convergence of neuroscience, pharmacology, and exercise physiology continues to build an exciting framework for the future of aging well—one where both molecular science and physical training are harnessed to preserve strength, resilience, and independence across the lifespan.
Together, rapamycin and resistance training may represent a synergistic approach—combining pharmacology and physiology—to maximize muscular strength, preserve independence, and extend healthspan.
- Haff, G., Triplett, TN. (2015). Essentials of strength training and conditioning (4 ed.). Human Kinetics.
- Stanfield, B., Kaeberlein, M., Leroux, B., Jones, J., Lucas, R., & Arroll, B. (2024). A single-center, double-blind, randomized, placebo-controlled, two-arm study to evaluate the safety and efficacy of once-weekly sirolimus (rapamycin) on muscle strength and endurance in older adults following a 13-week exercise program. Trials, 25(1), 642.
- Appeadu, M. K., & Bordoni, B. (2025). Falls and Fall Prevention in Older Adults. In StatPearls. StatPearls Publishing. Copyright © 2025, StatPearls Publishing LLC.
- Bouvard, B., Annweiler, C., & Legrand, E. (2021). Osteoporosis in older adults. Joint Bone Spine, 88(3), 105135.
- Larsson, L., Degens, H., Li, M., Salviati, L., Lee, Y. I., Thompson, W., Kirkland, J. L., & Sandri, M. (2019). Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol Rev, 99(1), 427-511.
- Carneiro, M. A. S., Franco, C. M. C., Silva, A. L., Castro, E. S. P., Kunevaliki, G., Izquierdo, M., Cyrino, E. S., & Padilha, C. S. (2021). Resistance exercise intervention on muscular strength and power, and functional capacity in acute hospitalized older adults: a systematic review and meta-analysis of 2498 patients in 7 randomized clinical trials. Geroscience, 43(6), 2693-2705.
- Reggiani, C., & Schiaffino, S. (2020). Muscle hypertrophy and muscle strength: dependent or independent variables? A provocative review. Eur J Transl Myol, 30(3), 9311.
- Sale, D. G. (1988). Neural adaptation to resistance training. Med Sci Sports Exerc, 20(5 Suppl), S135-145.
- Barry, B. K., & Carson, R. G. (2004). The consequences of resistance training for movement control in older adults. J Gerontol A Biol Sci Med Sci, 59(7), 730-754.
- Claudino, J. G., Afonso, J., Sarvestan, J., Lanza, M. B., Pennone, J., Filho, C. A. C., Serrão, J. C., Espregueira-Mendes, J., Vasconcelos, A. L. V., de Andrade, M. P., Rocha-Rodrigues, S., Andrade, R., & Ramirez-Campillo, R. (2021). Strength Training to Prevent Falls in Older Adults: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. J Clin Med, 10(14).
- Agarwal, S. K. (2012). Cardiovascular benefits of exercise. Int J Gen Med, 5, 541-545.
- Pinckard, K., Baskin, K. K., & Stanford, K. I. (2019). Effects of Exercise to Improve Cardiovascular Health. Front Cardiovasc Med, 6, 69.
- Ghorbanzadeh, M., Bakhtiari, A., Hajian-Tilaki, K., & Abbaszadeh-Amirdehi, M. (2025). Association of multidimensional frailty and dynapenia with fall risk in older adults. BMC Geriatr, 25(1), 442.
- Bohannon, R. W. (2019). Grip Strength: An Indispensable Biomarker For Older Adults. Clin Interv Aging, 14, 1681-1691.
- Lee, E. J., & Neppl, R. L. (2021). Influence of Age on Skeletal Muscle Hypertrophy and Atrophy Signaling: Established Paradigms and Unexpected Links. Genes (Basel), 12(5).
- Peterson, M. D., Rhea, M. R., Sen, A., & Gordon, P. M. (2010). Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev, 9(3), 226-237.
- Walker, S. (2021). Evidence of resistance training-induced neural adaptation in older adults. Experimental Gerontology, 151, 111408.
- Wilkinson, D. J., Piasecki, M., & Atherton, P. J. (2018). The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Research Reviews, 47, 123-132.
- Laplante, M., & Sabatini, D. M. (2009). mTOR signaling at a glance. J Cell Sci, 122(Pt 20), 3589-3594.
- Thoreen, C. C., & Sabatini, D. M. (2009). Rapamycin inhibits mTORC1, but not completely. Autophagy, 5(5), 725-726.
- Yu, K., & Toral-Barza, L. (2012). Biochemical and pharmacological inhibition of mTOR by rapamycin and an ATP-competitive mTOR inhibitor. Methods Mol Biol, 821, 15-28.
- Drummond, M. J., Fry, C. S., Glynn, E. L., Dreyer, H. C., Dhanani, S., Timmerman, K. L., Volpi, E., & Rasmussen, B. B. (2009). Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol, 587(Pt 7), 1535-1546.
- Ham, D. J., Börsch, A., Chojnowska, K., Lin, S., Leuchtmann, A. B., Ham, A. S., Thürkauf, M., Delezie, J., Furrer, R., Burri, D., Sinnreich, M., Handschin, C., Tintignac, L. A., Zavolan, M., Mittal, N., & Rüegg, M. A. (2022). Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle. Nature Communications, 13(1), 2025.
- Joseph, G. A., Wang, S. X., Jacobs, C. E., Zhou, W., Kimble, G. C., Tse, H. W., Eash, J. K., Shavlakadze, T., & Glass, D. J. (2019). Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia. Mol Cell Biol, 39(19).
- Selvarani, R., Mohammed, S., & Richardson, A. (2021). Effect of rapamycin on aging and age-related diseases-past and future. Geroscience, 43(3), 1135-1158.
- Lin, H., Salech, F., Lim, A., Vogrin, S., & Duque, G. (2022). The effect of rapamycin and its analogues on age-related musculoskeletal diseases: a systematic review. Aging Clin Exp Res, 34(10), 2317-2333.
- Schiaffino, S., & Mammucari, C. (2011). Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle, 1(1), 4.
- Ogasawara, R., Jensen, T. E., Goodman, C. A., & Hornberger, T. A. (2019). Resistance Exercise-Induced Hypertrophy: A Potential Role for Rapamycin-Insensitive mTOR. Exerc Sport Sci Rev, 47(3), 188-194.
- Rodriguez, J., Vernus, B., Chelh, I., Cassar-Malek, I., Gabillard, J. C., Hadj Sassi, A., Seiliez, I., Picard, B., & Bonnieu, A. (2014). Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci, 71(22), 4361-4371.
- Tang, H., Shrager, J. B., & Goldman, D. (2019). Rapamycin protects aging muscle. Aging (Albany NY), 11(16), 5868-5870.
- Tawfik, D. (2023). Rapamycin and Muscle Growth: A Review of the Glass Lab's use of Rapamycin to Reverse Sarcopenia and Anabolic Resistance. Healthspan.
- Bibee, K. P., Cheng, Y. J., Ching, J. K., Marsh, J. N., Li, A. J., Keeling, R. M., Connolly, A. M., Golumbek, P. T., Myerson, J. W., Hu, G., Chen, J., Shannon, W. D., Lanza, G. M., Weihl, C. C., & Wickline, S. A. (2014). Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. Faseb j, 28(5), 2047-2061.
- Thielen, S. C., Reusch, J. E. B., & Regensteiner, J. G. (2023). A narrative review of exercise participation among adults with prediabetes or type 2 diabetes: barriers and solutions. Front Clin Diabetes Healthc, 4, 1218692.
- Cooper, L. B., Mentz, R. J., Sun, J. L., Schulte, P. J., Fleg, J. L., Cooper, L. S., Piña, I. L., Leifer, E. S., Kraus, W. E., Whellan, D. J., Keteyian, S. J., & O'Connor, C. M. (2015). Psychosocial Factors, Exercise Adherence, and Outcomes in Heart Failure Patients: Insights From Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION). Circ Heart Fail, 8(6), 1044-1051.
- Garber C. E., Blissmer B., Deschenes M. R., Franklin B. A., Lamonte M. J., Lee I. M., Nieman D. C., Swain D. P. (2015). American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc, 43(7):1334-59.
Related studies