SGLT-2 Inhibitors: Do They Differ in Their Potential for Healthspan Extension?
A Single Mechanism, Multiple Benefits: By blocking the SGLT-2 transporter in the kidney, these drugs lower blood glucose independently of insulin, reduce sodium reabsorption, and trigger mild osmotic diuresis — a unified mechanism driving improvements in glycemic control, blood pressure, and cardiovascular outcomes.
Consistent Glucose Reduction Across the Class: Large randomized trials show all SGLT-2 inhibitors lower HbA1c by 0.5–1.0% on average, translating to a measurable reduction in cardiovascular and renal complications over time.
Caloric Loss and Weight Reduction: Each day, patients excrete 50–100 g of glucose, equivalent to roughly 200–400 kcal, leading to an average 2–3 kg weight loss over several months of therapy.
Cardiovascular Protection Beyond Diabetes: Major outcome trials — EMPA-REG, CANVAS, and DECLARE-TIMI 58 — show a 30–35% reduction in hospitalizations for heart failure and significant decreases in cardiovascular mortality, even in non-diabetic populations.
Renal Preservation Through Tubuloglomerular Feedback: Trials such as CREDENCE and DAPA-CKD demonstrate a 30–40% lower risk of kidney failure or sustained eGFR decline, linked to reduced intraglomerular pressure and improved sodium signaling.
Metabolic Shift Toward Ketone Utilization: SGLT-2 inhibitors promote a heart-protective energy substrate switch, increasing ketone body oxidation, which yields 10–20% more ATP per unit of oxygen than glucose — improving cardiac efficiency under stress.
Comparable Efficacy, Practical Differences: While all agents share core metabolic and organ-protective effects, bexagliflozin and empagliflozin stand out for high SGLT-2 selectivity, long half-lives, and favorable dosing; choice often depends on cost, renal thresholds, and formulary access rather than clinical performance.
Over the past few years, SGLT-2 inhibitors have evolved from niche diabetes drugs into one of the most promising therapeutic classes in modern medicine. Originally designed to help patients with type 2 diabetes lower blood glucose by promoting its excretion through the kidneys, these drugs have proven to do far more than regulate sugar. Large-scale clinical trials have shown that they protect the heart, preserve kidney function, and improve metabolic stability.
SGLT-2 inhibitors are a class of medications that includes canagliflozin, empagliflozin, dapagliflozin, ertugliflozin, and the newer bexagliflozin. These medications share a common mechanism but are not identical. Across numerous studies, these agents have shown remarkably consistent benefits in reducing cardiovascular events and slowing the progression of chronic kidney disease. Yet as their applications expand beyond disease treatment to potential roles in longevity and healthspan extension, a new question is emerging: do all SGLT-2 inhibitors work the same way when it comes to long-term metabolic protection and aging biology?
In this review, we explore the shared mechanisms that make SGLT-2 inhibitors so powerful, and examine whether meaningful differences between them could influence their impact on healthy aging. We’ll look at what unites the class, what sets certain agents apart, and how the use of SGLT-2 inhibitors in longevity science may represent a turning point in the science of extending healthspan.
How do SGLT-2 Inhibitors Work?
Sodium–glucose co-transporter 2 (SGLT-2) inhibitors represent a major advancement in modern medicine, significantly reshaping the management of multiple chronic conditions. Initially developed to treat the high blood sugar associated with type 2 diabetes (T2D), these drugs have been found to offer substantial cardiovascular and renal benefits, extending their use beyond glucose control.[1] As a result, SGLT-2 inhibitors are increasingly prescribed to patients regardless of whether they have diabetes. The key agents in this class include canagliflozin, empagliflozin, dapagliflozin, ertugliflozin, and the more recent addition, bexagliflozin. Their effectiveness and safety are supported by a robust body of evidence from large, international, placebo-controlled clinical trials. These studies consistently demonstrate that SGLT-2 inhibitors reduce hospitalizations for heart failure and slow the progression of chronic kidney disease, establishing them as cornerstone therapies in both cardiology and nephrology alongside their role in diabetes care.[2]
The therapeutic effects of SGLT-2 inhibitors result from their ability to block the SGLT-2 protein in the kidneys. This protein is located in the part of the kidney responsible for reabsorbing most of the glucose filtered from the blood. Under normal conditions, the SGLT-2 protein reabsorbs about 90 percent of the glucose, ensuring that it returns to the bloodstream instead of being lost in the urine. When SGLT-2 inhibitors attach to this protein, they reduce its ability to take glucose back into the blood. This causes more glucose to remain in the kidney and be excreted in the urine, a process called glycosuria.
Beyond their renal mechanism, what makes SGLT-2 inhibitors so effective is that they act independently of the pancreas’s beta cells. These are the cells that produce insulin, the hormone responsible for lowering blood sugar. In type 2 diabetes, beta-cell function gradually declines, reducing insulin output as the disease progresses. Most other glucose-lowering drugs rely on insulin secretion and therefore lose potency over time. SGLT-2 inhibitors bypass this limitation entirely: they lower blood sugar regardless of how well the pancreas is working. Another important advantage is that they do not stimulate excess insulin release, which means they rarely cause hypoglycemia when used alone or with other non-insulin-releasing therapies.
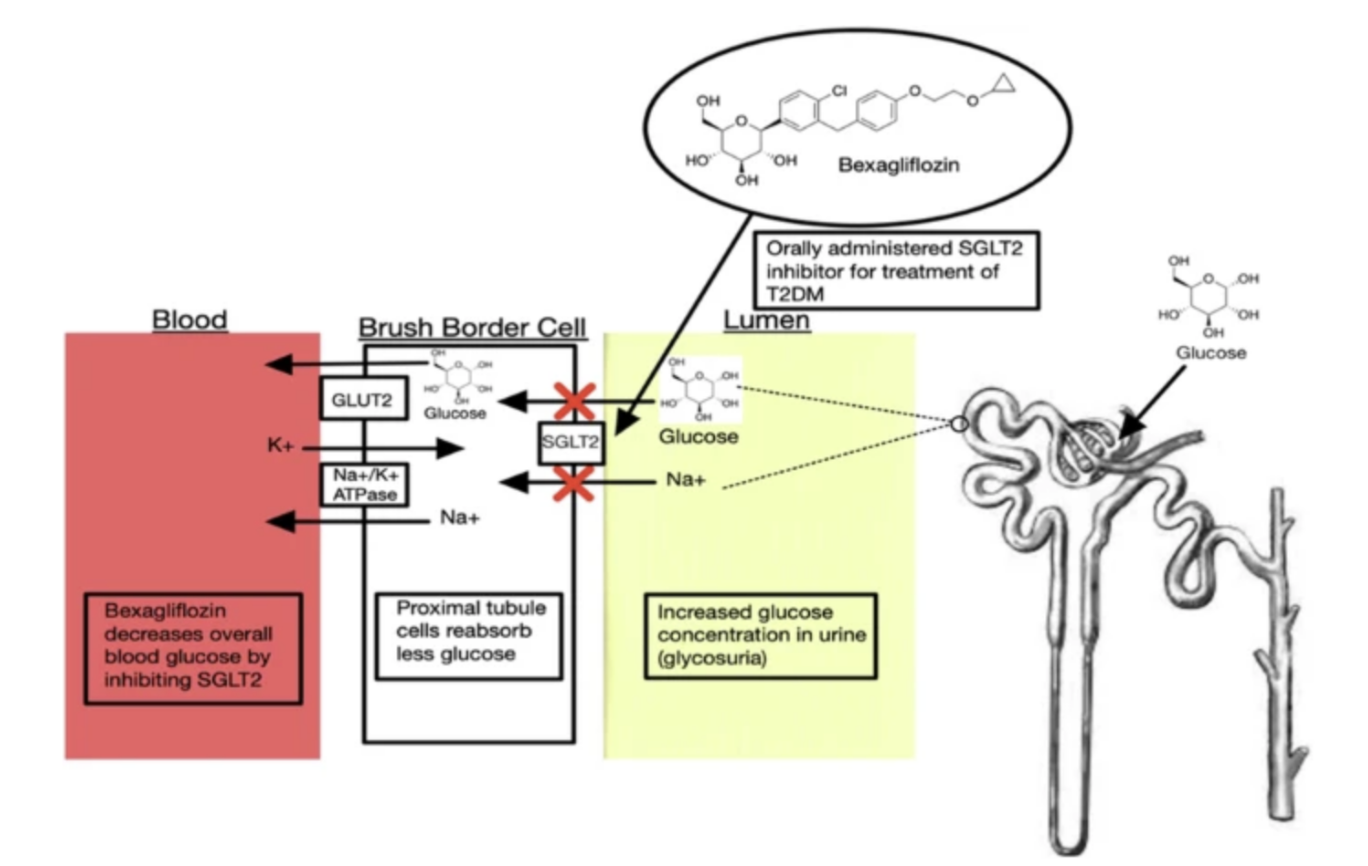
Figure 1: Mechanism of Action for Bexagliflozin, an SGLT-2 Inhibitor: This diagram illustrates the primary mechanism of action for bexagliflozin. In the proximal tubule cells of the kidney, the SGLT-2 transporter is normally responsible for reabsorbing approximately 90% of filtered glucose from the tubule lumen back into the blood. Bexagliflozin binds to and blocks the SGLT-2 transporter. This inhibition prevents glucose reabsorption and consequently lowers the overall blood glucose level.
Shared Class Benefits: How One Mechanism Drives Many Outcomes
How SGLT-2 Inhibitors Improve Glucose Regulation
One of the defining strengths of SGLT-2 inhibitors is the remarkable consistency of their benefits across all drugs in the class. This consistency supports the idea of a “class effect,” meaning that the improvements in heart, kidney, and metabolic outcomes come directly from the mechanism of blocking the SGLT-2 transporter, rather than from differences between individual drugs. This has been proven in multiple large randomized controlled trials, which were carefully designed studies meant to test both safety and effectiveness in thousands of patients.
The first and most obvious effect of these drugs is improved blood sugar control. Blood sugar is often tracked by a test called HbA1c, which measures the average level of glucose in the blood over the past two to three months. Across many major trials, SGLT-2 inhibitors consistently reduced HbA1c by about half a percent to one percent. This may sound modest, but it is both meaningful and reliable because long-term studies show that each one-percent drop in HbA1c can substantially reduce the likelihood of heart attack, stroke, and kidney failure.

Figure 2: Change in Hemoglobin A1c from Baseline: This graph illustrates the estimated average change in hemoglobin A1c, or HbA1c, from baseline after 24 weeks of treatment with bexagliflozin (an SGLT-2 inhibitor) at a dose of 20 mg compared to placebo. The pink bars represent patients receiving bexagliflozin, while the blue bars represent patients receiving a placebo. Across groups with varying levels of kidney function, bexagliflozin produced a meaningful and statistically significant reduction in HbA1c compared with placebo.
Besides glycemic management, an additional reason why these inhibitors are very effective is that they can influence body weight and blood pressure in important ways. By blocking glucose reabsorption in the kidney, they cause glucose to spill into the urine. Patients can lose between 50 and 100 grams of glucose every day, which is a significant amount of calories. Over time, this steady loss can translate into a body weight reduction of about 2 to 3 kilograms on average. Alongside glucose, sodium is also lost in the urine because sodium and glucose are normally reabsorbed together. The loss of sodium pulls water with it, which slightly reduces blood volume. This combination leads to a modest but consistent drop in systolic blood pressure, usually between 3 and 5 millimeters of mercury.
Patients can lose between 50 and 100 grams of glucose every day, which is a significant amount of calories. Over time, this steady loss can translate into a body weight reduction of about 2 to 3 kilograms on average.
How SGLT-2 Inhibitors Provide Cardioprotection
One of the most remarkable discoveries about SGLT-2 inhibitors is their unexpected ability to protect the heart. Originally, large cardiovascular trials were required for new diabetes drugs to prove they did not increase the risk of heart attacks or strokes. The EMPA-REG OUTCOME trial, which studied empagliflozin in people with type 2 diabetes and high cardiovascular risk, went far beyond that expectation. It showed that patients not only had fewer hospitalizations for heart failure but also lived longer, with a marked reduction in cardiovascular death. [5] The CANVAS Program with canagliflozin and the DECLARE-TIMI 58 trial with dapagliflozin confirmed these results. Across these studies, hospitalizations for heart failure dropped by about 30 to 35 percent. [6] Importantly, these benefits appeared early, were seen in both types of heart failure, and even extended to patients without diabetes.
Scientists have worked to explain how this protection arises. One mechanism is that reducing blood volume eases strain on the heart. Another is that the heart begins to shift the type of fuel it uses for energy. Under normal conditions, the heart uses both glucose and fatty acids. In stressed states like heart failure, this balance becomes inefficient. SGLT-2 inhibitors seem to push the heart toward using ketone bodies, which are small molecules produced when fat is broken down. Ketone bodies provide energy more efficiently than glucose or fat, meaning the heart can produce more work with less oxygen. This metabolic shift may explain why patients often feel better and have improved exercise capacity. In addition, the drugs reduce inflammation and scarring in the heart tissue, which helps preserve its structure and function.[7]
One mechanism is that reducing blood volume eases strain on the heart. Another is that the heart begins to shift the type of fuel it uses for energy. Under normal conditions, the heart uses both glucose and fatty acids. In stressed states like heart failure, this balance becomes inefficient. SGLT-2 inhibitors seem to push the heart toward using ketone bodies, which are small molecules produced when fat is broken down. Ketone bodies provide energy more efficiently than glucose or fat, meaning the heart can produce more work with less oxygen.
How SGLT-2 Inhibitors Preserve Renal Health
The kidney benefits of SGLT-2 inhibitors are also significant. Kidney function is usually measured by estimated GFR. This number reflects how much blood the kidneys filter each minute, showing how well they are clearing waste from the body. In chronic conditions like diabetes, GFR tends to fall steadily over the years, eventually leading to kidney failure. Another important marker of kidney damage is albuminuria, which means that albumin, the main protein in the blood, is leaking into the urine. Normally, the kidney filters should keep albumin inside the bloodstream. Its presence in urine is an early warning sign of injury to the kidney’s filtering units. Recent studies have shown that treatment with SGLT-2 inhibitors slows the decline of eGFR and lowers albuminuria. Dedicated kidney trials, such as CREDENCE and DAPA-CKD, tested these medications in patients with already reduced kidney function and albuminuria.[8] Both studies confirmed that SGLT-2 inhibitors prevent further damage and significantly lower the risk of kidney failure.[9]
Much of the kidney protection offered by SGLT-2 inhibitors comes from how they rebalance pressure inside the kidney’s filtering units through a process called tubuloglomerular feedback.
Normally, the kidneys reabsorb most of the filtered glucose and sodium early in the tubule. When SGLT-2 is blocked, less of this reabsorption happens, and more sodium reaches a group of sensing cells known as the macula densa. These cells sit next to the kidney’s tiny filters, called glomeruli, and act as pressure regulators. Sensing the higher sodium levels, the macula densa sends a signal that causes the small blood vessel feeding the glomerulus to tighten slightly. This narrowing reduces internal pressure in the glomerulus, which helps protect it from long-term stress and injury.
Choosing the Right SGLT-2 Inhibitor: Are They All the Same?
While all SGLT-2 inhibitors share a common kidney-based mechanism and deliver broad metabolic and cardiovascular benefits, subtle pharmacologic differences distinguish one agent from another. These differences affect how selectively each drug targets the SGLT-2 transporter, how long it remains active in the body, and how it is processed and cleared.
The most meaningful variation lies in selectivity between the SGLT-2 and SGLT-1 transporters. SGLT-1 primarily absorbs glucose in the intestine and contributes to a small portion of renal glucose reuptake. Drugs such as empagliflozin, dapagliflozin, ertugliflozin, and bexagliflozin show high SGLT-2 selectivity, meaning their effects are focused on the kidney, where they promote glucose and sodium excretion without altering intestinal absorption. [11]
Among these, bexagliflozin stands out as a particularly efficient and cost-effective agent. Its high selectivity, long plasma half-life, and consistent once-daily dosing support durable glycemic control even in patients with reduced kidney function. Studies also suggest modest improvements in blood pressure, body weight, and key cardiovascular markers. Together, these traits make bexagliflozin an appealing option for sustained metabolic health and potential applications in healthy aging. By comparison, canagliflozin remains one of the best-studied SGLT-2 inhibitors and has robust evidence for kidney and cardiovascular protection. However, its high cost and limited availability have reduced its accessibility and practicality for long-term use.
Despite these nuances, ultimately, all approved SGLT-2 inhibitors provide the same core clinical benefits. These include lowering blood sugar, providing cardiovascular protection, and protecting kidney function. Because these benefits are shared across the class, the choice of a specific drug is often guided by practical considerations such as cost, availability on insurance formularies, or specific guidance about starting treatment in patients with reduced kidney function.
At first, this shift can cause a small, temporary drop in estimated GFR. But rather than being harmful, this early dip reflects the kidney’s return to a healthier filtration pressure. Over time, that normalization preserves kidney structure, slows chronic damage, and extends overall kidney function.
- Padda, I. S., Mahtani, A. U., & Parmar, M. (2023). Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. PubMed; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK576405/
- Hsia, D. S., Grove, O., & Cefalu, W. T. (2017). An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Current opinion in endocrinology, diabetes, and obesity, 24(1), 73–79. https://doi.org/10.1097/MED.0000000000000311
- France, N. L., & Shirley, M. (2024). Bexagliflozin in type 2 diabetes: a profile of its use. Drugs & Therapy Perspectives, 40(7), 241–249. https://doi.org/10.1007/s40267-024-01098-1
- Allegretti, A. S., Zhang, W., Zhou, W., Thurber, T. K., Rigby, S. P., Bowman-Stroud, C., Trescoli, C., Serusclat, P., Freeman, M. W., & Halvorsen, Y.-D. C. (2019). Safety and Effectiveness of Bexagliflozin in Patients With Type 2 Diabetes Mellitus and Stage 3a/3b CKD. American Journal of Kidney Diseases, 74(3), 328–337. https://doi.org/10.1053/j.ajkd.2019.03.417
- Fitchett, D., Zinman, B., Wanner, C., Lachin, J. M., Hantel, S., Salsali, A., Johansen, O. E., Woerle, H. J., Broedl, U. C., Inzucchi, S. E., & EMPA-REG OUTCOME® trial investigators (2016). Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. European heart journal, 37(19), 1526–1534. https://doi.org/10.1093/eurheartj/ehv728
- Neal, B., Perkovic, V., Mahaffey, K. W., de Zeeuw, D., Fulcher, G., Erondu, N., Shaw, W., Law, G., Desai, M., & Matthews, D. R. (2017). Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. New England Journal of Medicine, 377(7), 644–657. https://doi.org/10.1056/nejmoa1611925
- Tanawan Kongmalai, Phorntida Hadnorntun, Pattara Leelahavarong, Pinkawas Kongmalai, Varalak Srinonprasert, Srisakul Chirakarnjanakorn, Usa Chaikledkaew, McKay, G., Attia, J., & Ammarin Thakkinstian. (2023). Comparative cardiovascular benefits of individual SGLT2 inhibitors in type 2 diabetes and heart failure: a systematic review and network meta-analysis of randomized controlled trials. Frontiers in Endocrinology, 14. https://doi.org/10.3389/fendo.2023.1216160
- Perkovic, V., Jardine, M. J., Neal, B., Bompoint, S., Heerspink, H. J. L., Charytan, D. M., Edwards, R., Agarwal, R., Bakris, G., Bull, S., Cannon, C. P., Capuano, G., Chu, P.-L., de Zeeuw, D., Greene, T., Levin, A., Pollock, C., Wheeler, D. C., Yavin, Y., & Zhang, H. (2019). Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New England Journal of Medicine, 380(24), 2295–2306. https://doi.org/10.1056/nejmoa1811744
- Yau, K., Dharia, A., Alrowiyti, I., & Cherney, D. Z. I. (2022). Prescribing SGLT2 Inhibitors in Patients With CKD: Expanding Indications and Practical Considerations. Kidney international reports, 7(7), 1463–1476. https://doi.org/10.1016/j.ekir.2022.04.094
- Azizogli, A. R., Vitti, M. R., Mishra, R., Osorno, L., Heffernan, C., & Kumar, V. A. (2023). Comparison of SGLT1, SGLT2, and Dual Inhibitor biological activity in treating Type 2 Diabetes Mellitus. Advanced therapeutics, 6(12), 2300143. https://doi.org/10.1002/adtp.202300143
- Khalid, Z., & Patel, P. (2024). Canagliflozin. PubMed; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK603733/
- Carbone, S., & Dixon, D. L. (2019). The CANVAS Program: implications of canagliflozin on reducing cardiovascular risk in patients with type 2 diabetes mellitus. Cardiovascular diabetology, 18(1), 64. https://doi.org/10.1186/s12933-019-0869-2
- Wada, T., Mori-Anai, K., Takahashi, A., Matsui, T., Inagaki, M., Iida, M., Maruyama, K., & Tsuda, H. (2022). Effect of canagliflozin on the decline of estimated glomerular filtration rate in chronic kidney disease patients with type 2 diabetes mellitus: A multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase III study in Japan. Journal of diabetes investigation, 13(12), 1981–1989. https://doi.org/10.1111/jdi.13888
- Sokolov, V., Yakovleva, T., Chu, L., Tang, W., Greasley, P. J., Johansson, S., Peskov, K., Helmlinger, G., Boulton, D. W., & Penland, R. C. (2020). Differentiating the Sodium-Glucose Cotransporter 1 Inhibition Capacity of Canagliflozin vs. Dapagliflozin and Empagliflozin Using Quantitative Systems Pharmacology Modeling. CPT: pharmacometrics & systems pharmacology, 9(4), 222–229. https://doi.org/10.1002/psp4.12498
Related studies