Epitalon is a multi-pathway geroprotector: Unlike most interventions that hit a single hallmark of aging, Epitalon acts on five: telomere maintenance, epigenetic regulation, oxidative stress resilience, immune recalibration, and circadian rhythm restoration, making it one of the broadest-reaching peptides studied in longevity science.
Telomerase activation is its signature mechanism: Epitalon can activate telomerase and lengthen telomeres in human cells, extending their replicative lifespan beyond the Hayflick limit, while preserving youthful morphology and function, though this benefit must be weighed against potential cancer risks.
It remodels the epigenome and protects DNA: By binding to promoter regions and loosening chromatin structure, Epitalon may restore youthful gene expression patterns, enhance DNA repair, and reduce mutation load, positioning it as a precision gene-expression modulator rather than a blunt genetic tool.
It strengthens intrinsic antioxidant defenses: Epitalon boosts key antioxidant enzymes through Nrf2 activation, reduces oxidative damage markers, preserves mitochondrial integrity, and supports tissue resilience, effects observed from skin fibroblasts to brain and reproductive cells.
Immune effects are restorative, not overstimulating: It rebalances T-cell ratios, upregulates IL-2 in aged tissues, and improves neuroimmune integration, suggesting a targeted recalibration of immune tone rather than indiscriminate “boosting.”
It restores circadian and hormonal rhythms: By stimulating melatonin synthesis, protecting pineal structure, and re-entraining clock genes, Epitalon may counter the widespread circadian drift of aging, with implications far beyond sleep, touching immunity, metabolism, and neuroprotection.
The promise is real, but clinical readiness is not: Preclinical and early human data are compelling, but without large-scale trials, validated delivery methods, and long-term safety data, Epitalon remains an experimental peptide, one to watch closely, but not yet ready for mainstream clinical adoption.
Introduction to Epitalon: What Is It? And Why Are Longevity Experts Watching Closely?
While most anti-aging interventions target just one hallmark of aging, Epitalon targets five [1]. This synthetic peptide is quietly redefining how we think about multi-pathway longevity therapeutics. While most longevity interventions target single hallmarks of aging, like NAD+ boosters for mitochondrial support [2] or senolytics to clear senescent cells [3], Epitalon’s appeal lies in its multi-dimensional role in aging biology. It targets not just one, but several core mechanisms of aging simultaneously: telomere maintenance, epigenetic regulation, oxidative stress resilience, immune recalibration, and circadian rhythm restoration [1]. In an era where the limitations of single-target interventions are becoming clear, Epitalon’s systems-level influence is attracting renewed attention from scientists and advanced longevity practitioners alike.
And unlike other molecules in longevity science, it’s been tested across an unusually broad range of models: from fruit flies to non-human primates, and from human fibroblasts to frog embryos.
In the rapidly evolving field of geroscience, few peptides have stirred as much curiosity and cautious optimism as Epitalon. Often labeled in longevity circles as the “anti-aging peptide,” it’s far more than a fleeting trend. Epitalon is a synthetic tetrapeptide (Ala-Glu-Asp-Gly; AEDG) (Fig. 1), structurally modeled on Epithalamin, a natural extract derived from the pineal gland of cattle [1, 4].
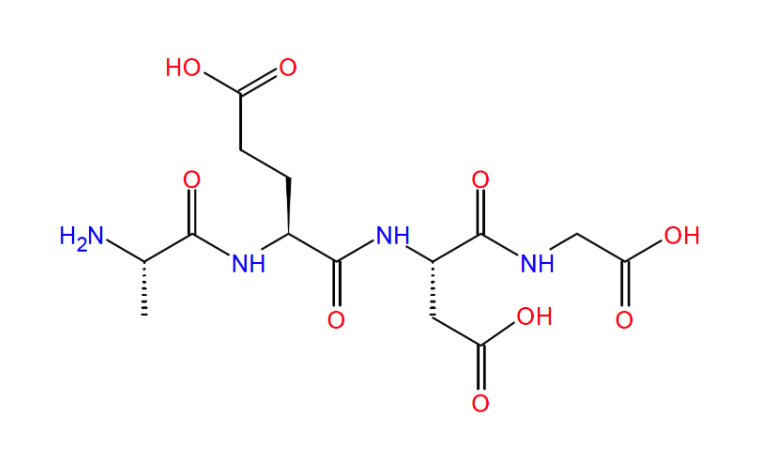
Figure 1: Understanding Epitalon’s simple tetrapeptide structure is key for appreciating its selective DNA interactions and potential ease of synthesis for clinical-grade formulations. Image from the paper of Araj et. al., 2025.
The pineal gland (Fig. 2) is a small, pea-shaped endocrine organ nestled deep within the epithalamus, between the two hemispheres of the brain, just above the midbrain tectum and behind the third ventricle. Though tiny, it plays a significant role in regulating circadian rhythms through the production of melatonin. It lies adjacent to key structures such as the pituitary gland, optic chiasm, and ventricular system, which circulates cerebrospinal fluid (CSF). Often referred to as the "third eye" due to its light-sensitive regulatory role, the pineal gland serves as the body’s internal timekeeper, synchronizing hormonal rhythms with environmental light cues.
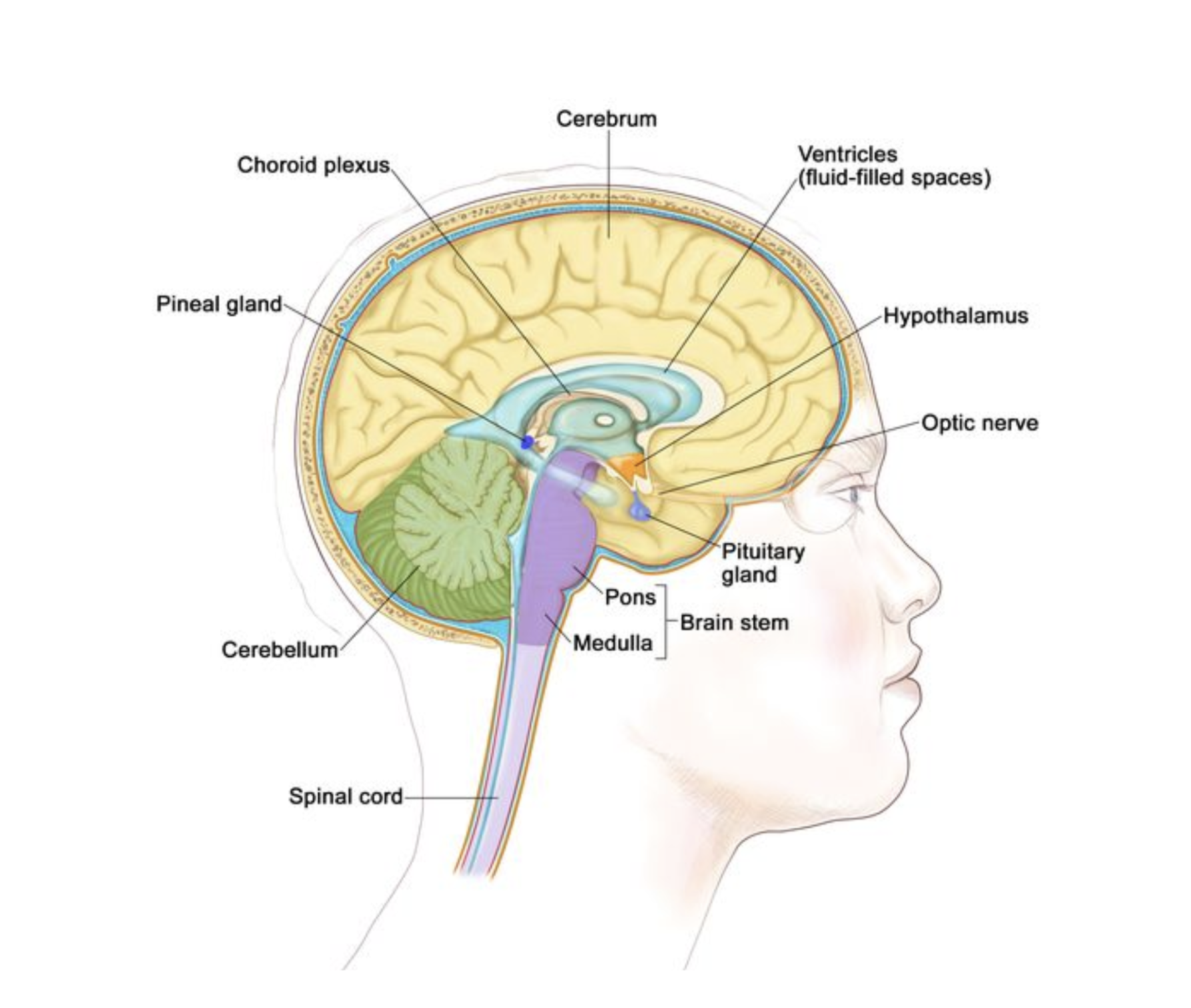
Figure 2: The pineal gland’s anatomical position within the neuroendocrine hub explains its outsized influence on circadian, immune, and hormonal systems, making Epitalon’s tissue-specific action particularly significant. Image from https://www.cancer.gov/publications/dictionaries/cancer-terms/def/pineal-gland, accessed on 30 July 2025.
Melatonin is well-known for its role in sleep regulation, but it also influences immune function, oxidative stress, and even cellular repair mechanisms [6].
Epitalon has become a focal point in longevity research. For decades, its physiological relevance remained speculative, as the peptide had not yet been identified in human tissue. That changed in 2017, when researchers confirmed the presence of Epitalon in native pineal gland extracts, a discovery that validated its endogenous origin and helped explain its overlap with, but also differences from, Epithalamin [7]. While both peptides share similar geroprotective properties, Epitalon appears to differ in intensity and spectrum of action, possibly due to its structure or formulation [8, 9].
Today, Epitalon is usually studied in its free-base form. Some research also explores acetate or trifluoroacetate (TFA) salt forms. Despite its peptide nature, which often poses bioavailability challenges, Epitalon has been reported in some sources to exhibit resistance to hydrolysis, suggesting potential for oral uptake under specific conditions [1,4, 10]. Nevertheless, the vast majority of published studies rely on subcutaneous injection routes to ensure consistent systemic absorption and biological effect [11].
Epitalon’s appeal lies in its unusually broad mechanistic reach: it can activate telomerase and support telomere maintenance in human cells [12,13,14, 15], modulate gene expression epigenetically by influencing chromatin accessibility and promoter interactions [4], enhance tissue repair in disease contexts such as diabetic retinopathy by reducing oxidative stress and EMT while improving wound healing in retinal cells [16], bolster redox homeostasis by upregulating endogenous antioxidant defenses and lowering DNA-damage markers [1], rebalance immune tone via IL-2 upregulation [17] and shifts in CD4+/CD8+ profiles [18], and even reset circadian biology by tuning core clock genes and melatonin dynamics [19, 20].
In short, Epitalon doesn’t act on one aging lever; it acts on many. This makes it both exciting and difficult to pin down. It doesn’t “cure” aging (nothing does), but it appears to modulate several pathways that otherwise accelerate it.
Interest in Epitalon has grown in part due to its visibility on biohacking forums, and partially due to the resurgence of peptide therapeutics in both academic and clinical spaces. The publication of high-quality mechanistic studies, coupled with small human trials showing functional endocrine and immune effects, has elevated the peptide from fringe curiosity to a serious candidate in the longevity toolkit.
But for all its promise, Epitalon still occupies a grey zone: scientifically fascinating, clinically provocative, but not yet fully understood. That’s why this article aims to dissect the evidence with precision, walk through its mechanistic layers, and separate signals from speculation.
While its mechanisms are complex and wide-ranging, questions of delivery and dosing remain front of mind for both researchers and clinicians. Understanding how Epitalon is administered provides the practical foundation before we dive into biology.
Delivery, Dosing, and Practical Considerations: What We Know (and Don’t)
Before exploring Epitalon’s cellular mechanisms, it’s important to ask a practical question: how is this peptide actually delivered, and what do we know about dosing?
As a peptide, Epitalon faces major pharmacokinetic challenges, particularly poor oral bioavailability due to enzymatic degradation in the gut. This has led researchers to explore alternative delivery routes that maximize cellular uptake, stability, and systemic reach, while minimizing degradation.
Across most animal and human studies, two primary routes dominate [1]:
- Subcutaneous injection: Used in numerous rodent studies (typically 0.1–10 µg/mouse/day) and in human trials (commonly 0.5–1 mg/day), especially in circadian and immune studies.
- Intranasal administration: Employed in studies on hypothalamic IL-2 expression and cognitive outcomes, leveraging the olfactory bulb for CNS entry.
These routes bypass first-pass metabolism, offering more reliable delivery to target tissues, especially in neuroendocrine and immunomodulatory applications.
For Epitalon to move from niche biohacking to clinical application, a viable delivery system is non-negotiable. Sublingual sprays or transdermal patches must be developed to make them realistic in clinical workflows.
In models of retinal degeneration, Epitalon has been delivered via parabulbar injection, a technique that places the compound near the posterior eye to maximize local availability [64, 65]. While effective in rats, this delivery method is unlikely to be practical or scalable in clinical longevity settings.
To address systemic uptake limitations, researchers have explored dendrimer-conjugated Epitalon. These tree-like nanostructures improve peptide penetration through cell membranes. In silico and in vitro studies show enhanced bioavailability and intracellular access, though no in vivo human studies have yet validated this method [66]. This route offers potential for future oral or transdermal formulations, but more research is needed before real-world use.
Despite its growing popularity in biohacking circles and advanced longevity practitioners, Epitalon’s long-term safety remains unclear:
- No large-scale toxicology or pharmacokinetic studies in humans have been published.
- The peptide exists in eight stereoisomeric forms, but only the all-L form (natural configuration) has been studied. The effects and potential toxicity of other forms remain untested.
Until stereoisomer-specific studies and formal pharmacovigilance data are available, any long-term or high-dose use carries unknown risk.
If one were to extrapolate from existing human trials:
- Dose: 0.5–1 mg per day
- Route: Sublingual or subcutaneous
- Duration: Up to 20 days (as used in circadian gene studies)
Yet these regimens should not be considered prescriptive or approved protocols. Without validated biomarkers of efficacy, real-world results remain anecdotal at best.
For peptides like Epitalon to move from lab to clinic, the delivery method matters as much as the mechanism. A molecule that can activate telomerase or modulate circadian genes is only useful if it reaches its target intact. As it stands, Epitalon’s pharmacological profile is promising but incomplete, raising key questions about long-term safety, optimal dosing, and how to harness its effects in a reliable, standardized way.
With these practical considerations in mind, we can turn to the cellular level where some of the most striking evidence lies in Epitalon’s effects on telomeres.
Epitalon and Telomere Length: The Anti-Aging Mechanism Everyone’s Talking About
If aging had a clock, telomeres would be the ticking hands and telomerase, the mechanism that resets it.
Telomeres are repetitive DNA sequences (TTAGGG/AATCCC) that cap the ends of chromosomes, protecting genetic material during cell division. But each time a cell divides, these protective caps erode slightly, like the tips of shoelaces fraying over time. Eventually, when telomeres become critically short, the cell enters senescence: an irreversible state of growth arrest linked to aging and disease [22].
This attrition is what scientists call the Hayflick limit, named after Leonard Hayflick, who discovered that normal human cells divide roughly 40–60 times before stopping [23]. The enzyme telomerase can restore these caps, but in most somatic cells, telomerase is switched off after birth. This makes activating telomerase in adult tissues a tantalizing yet controversial strategy in longevity science.
One of Epitalon’s most compelling features is its ability to reactivate telomerase in human cells. Epitalon’s ability to reactivate telomerase has been demonstrated using the Telomeric Repeat Amplification Protocol (TRAP) assay in two key cell models:
- HeLa cells (telomerase-positive cancer cell line) and
- Human fetal lung fibroblasts (602/17).
In both cases, Epitalon significantly enhanced telomerase activity, directly contributing to telomere elongation during the G1 phase of the cell cycle [25] [26].
Mechanistically, Epitalon’s effect appears to involve a direct interaction with promoter regions of the telomerase gene, where it binds specific DNA sequences (such as ATTTC motifs), potentially modulating transcriptional activity. This epigenetic action is supported by findings showing Epitalon’s capacity to penetrate cell nuclei and influence chromatin structure, making telomerase genes more accessible for activation. These mechanisms together suggest that Epitalon doesn’t merely act as a passive support molecule but serves as a molecular signal that can unlock telomerase expression, promoting cellular longevity.
But it wasn’t just enzyme activity that changed.
When fetal fibroblasts were treated with Epitalon over multiple passages, they were able to bypass the Hayflick limit, dividing beyond 44 passages, compared to just 34 in untreated controls [26]. These cells not only retained mitotic capacity, but also maintained a more youthful morphology and gene expression profile, indicating real preservation of cellular “youth”.
In another human study, Epitalon was applied to PHA-stimulated lymphocytes from adult donors aged 25 to 88 years. Epitalon can induce the expression of telomerase enzyme components and increase telomerase activity. In some studies, telomere length rose by an average of 33.3%, though results varied between individuals, likely due to differences in baseline telomerase activity, cellular age, and other metabolic factors [4, 14].
Epitalon’s influence on telomerase isn't limited to aging models. In bovine cumulus–oocyte complexes, the peptide not only activated telomerase but also enhanced TERT protein localization, a key driver of telomere repair during early development [15]. This suggests that Epitalon may have broader applications, not only in delaying aging, but in enhancing reproductive biology and embryonic viability.
These findings collectively point to Epitalon as a functional telomerase modulator, capable of extending telomeres, delaying replicative senescence, and maintaining cellular health across multiple biological systems.
While it's too early to call Epitalon a “fountain of youth,” its ability to influence telomere dynamics puts it in rare company. Only a handful of other compounds show similar potential, including resveratrol, which activates sirtuins linked to telomere preservation; astragaloside IV from Astragalus root, known to stimulate telomerase activity ; Coenzyme Q10 (CoQ10), which supports mitochondrial health and indirectly stabilizes telomere length through reduced oxidative stress; curcumin, which helps maintain genomic stability and modulates telomere-associated proteins; and omega-3 fatty acids, which are associated with slower telomere attrition via anti-inflammatory and antioxidant effects.
Telomere attrition is linked not just to aging, but to cardiovascular disease, immune decline, type 2 diabetes, and neurodegeneration [28,29, 30]. By restoring telomerase activity in targeted ways, Epitalon may help preserve:
- Tissue regeneration capacity
- Stem cell viability
- Immune system responsiveness
- Genomic integrity
Of course, telomerase activation is a double-edged sword: while it promotes healthy cell division, it can also fuel unchecked growth in malignant cells. That’s why context, dose, and delivery all matter and why the rest of this article will explore Epitalon’s effects beyond telomeres.
In the next section, we’ll look at how Epitalon interacts with the epigenetic code, and why that might be just as important for aging as telomeres themselves.
DNA Protection and Epigenetics: How Epitalon May Slow Down the Aging Clock
While telomeres mark the visible end of the cellular lifespan, the epigenome serves as its regulatory backbone. Every cell in your body carries the same DNA sequence, but it’s the epigenetic instructions that determine which genes are switched on or off, when they’re activated, and how intensely they’re expressed. This dynamic regulation shapes everything from cellular identity to tissue repair and aging trajectories. Crucially, epigenetics acts as the interface between your genes and your environment, meaning that factors like stress, diet, sleep, and toxin exposure can alter gene expression without changing the underlying DNA code [31]. These changes can be reversible or persistent, and they play a central role in how diseases develop or are prevented across the lifespan.
Over time, the genome becomes increasingly dysregulated: DNA regions that should be silent become noisy, protective genes are switched off, and chromatin, the scaffold of DNA wrapped around histones (imagine them like packaging bundles), becomes condensed and brittle, losing its flexibility.
This is where Epitalon steps into a more subtle but powerful role: modulating gene expression by physically interacting with DNA and chromatin structures.
In a series of fluorescence microscopy and biochemical studies, Epitalon was shown to enter the nuclei of human HeLa cells and bind selectively to DNA regions rich in CAG and ATTTC repeats, sequences that are common within promoter regions of the telomerase gene [1,32,33]. These promoter regions act like dimmer switches for gene expression, and by binding here, Epitalon appears to gently dial up telomerase gene transcription without altering the DNA itself. This suggests that Epitalon may not just restore telomere length through enzymatic activation, but it may actually reprogram the transcriptional readiness of cells, shifting their expression profiles toward a more youthful state.
But gene expression is not only about DNA sequences: it’s about how tightly that DNA is packaged. As we age, chromatin tends to become heterochromatic: densely packed, transcriptionally silent, and resistant to repair [34]. In vitro studies on aged human lymphocytes found that Epitalon reversed this heterochromatinization, increasing chromatin plasticity and making DNA more accessible to transcription factors.
In silico molecular docking further supports this, showing that Epitalon has a high binding affinity for specific histone subtypes, H1/3 and H1/6, which play a regulatory role in chromatin compaction and accessibility [4].
This is not a blunt-force genomic intervention. Rather, Epitalon may act like a molecular locksmith, loosening tightly wound regions of DNA, allowing previously silenced genes, such as those involved in antioxidant defenses or DNA repair, to reawaken.
In addition to chromatin remodeling, Epitalon exhibits antimutagenic effects. In cultured human cells exposed to heavy metals (Zn, Co, Ni) known to induce DNA breaks and strand instability, Epitalon significantly reduced the extent of genotoxic damage, acting as a protective agent [36].
Moreover, the peptide was shown to lower the melting temperature of DNA by 41°C in vitro. While that may sound destabilizing, it points to a conformational shift in DNA duplex architecture that could facilitate transcription while preventing hardening of the genome that often accompanies age and oxidative stress [37].
While telomerase gets the headlines, epigenetic erosion is the silent driver of many age-related diseases, from Alzheimer’s to cancer to immunosenescence. If a cell’s DNA is its operating system, then the epigenome is the software. Aging corrupts the code. Epitalon appears to rewrite parts of this script, at least in vitro, nudging cells toward more resilient states of function.
Its ability to intervene upstream at the level of transcriptional regulation and chromatin architecture marks it as more than a telomerase hack. It may be one of the first peptides shown to act as a selective gene expression modulator, without the off-target chaos of pharmacological epigenetic drugs.
In the next section, we’ll look at how Epitalon translates these genomic effects into cellular protection, specifically how it bolsters our antioxidant defenses and shields our DNA from oxidative damage.
Oxidative Stress and Inflammation: Epitalon’s Antioxidant and Anti-Mutagenic Actions
At the cellular level, aging isn’t just about time - it’s about damage. Specifically, the relentless wear-and-tear from reactive oxygen species (ROS) and chronic low-grade inflammation. Together, these forces erode mitochondrial function, damage DNA, and slowly push cells toward dysfunction and death [38, 39].
Reactive oxygen species are unstable molecules produced during normal metabolism, but in excess, they wreak havoc. These free radicals damage DNA, proteins, and lipids, leading to cellular dysfunction and senescence. Over time, this cumulative oxidative stress impairs mitochondrial function, disrupts cell signaling, and promotes inflammation - all hallmarks of biological aging. High ROS levels are linked to diseases like Alzheimer’s, cardiovascular disease, and cancer. Slowing aging requires more than scavenging ROS; it requires restoring cellular control over redox balance, which peptides like Epitalon may help achieve.
In this context, Epitalon shows surprising breadth, not only as a gene expression modulator, but also as an active defender against oxidative stress and apoptosis. It does so by activating endogenous antioxidant systems and restoring mitochondrial integrity, which are both cornerstones of healthy aging.
Epitalon has been shown to significantly increase the expression of key antioxidant enzymes:
- Superoxide dismutase (SOD-1)
- Catalase
- NAD(P)H quinone dehydrogenase 1 (NQO1)
These effects are mediated via the Keap1/Nrf2 signaling pathway [40, 41]: a master regulator of cellular redox balance. Activation of this pathway increases the cell’s ability to neutralize ROS before they can damage proteins, lipids, or DNA.
This isn’t a superficial tweak. In human skin fibroblasts, Nrf2-driven antioxidant enzyme expression translated into:
- Reduced intracellular ROS
- Improved mitochondrial membrane potential
- Protection against senescence-associated cell damage [36]
In human gingival and periodontal stem cells, Epitalon significantly reduced aging biomarkers such as p16 and p21, decreased ROS levels, and preserved mitochondrial function, even under stress [42]. These findings suggest potential relevance not just for longevity, but also for regenerative medicine and age-related tissue repair.
In a study examining the effects of γ-irradiation on rat duodenum tissue, intraperitoneally administered Epitalon inhibited cell proliferation but did not enhance post-irradiation repair. The authors noted that this proliferation-inhibiting effect could have potential utility in anti-tumor applications, though further studies are needed to confirm its relevance in oncology contexts [43].
Epitalon’s antioxidant and anti-mutagenic actions aren’t species-limited. In Drosophila melanogaster, the peptide reduced mitochondrial and cytosolic ROS levels, along with lipid peroxidation markers such as Schiff bases and hydroperoxides. These reductions translated into enhanced locomotor activity and lifespan extension in treated flies [44].
In CBA mice, long-term administration of Epitalon preserved brain and liver mitochondrial ultrastructure, even after irradiation, suggesting real structural protection at the organ level [45].
In mouse oocytes, Epitalon significantly reduced levels of γH2AX, a marker of DNA double-strand breaks, and prevented apoptosis [46]. Given the high oxidative sensitivity of reproductive tissues, this points toward applications in both aging and fertility preservation.
Oxidative stress and inflammation are tightly linked to virtually every degenerative condition associated with aging: from atherosclerosis and cancer to neurodegeneration and sarcopenia. The capacity of Epitalon to activate intrinsic defenses, reduce damage markers, and stabilize mitochondrial function positions it not just as an antioxidant, but as a true cellular protectant. And unlike direct antioxidant supplements (e.g., vitamin C or E), which often fail in clinical trials due to poor bioavailability or paradoxical pro-oxidant effects, Epitalon works endogenously, stimulating the body's systems with surgical precision.
As we’ll explore next, these protective mechanisms intersect with yet another aging pathway: immune senescence. In the following section, we’ll examine how Epitalon may rejuvenate immune function by modulating cytokines and restoring youthful T cell profiles.
Immune Function and Inflammation: Epitalon’s Role as a Cytokine Modulator
As we age, the immune system undergoes a progressive decline in function, a phenomenon known as immunosenescence. This process is marked by chronic low-grade inflammation (“inflammaging”), diminished adaptive immunity, and a skewed CD4+/CD8+ T cell ratio, leaving older adults more vulnerable to infection, cancer, and poor vaccine responses [47].
Peptides like Epitalon may offer a targeted immune recalibration, especially for aging or immunocompromised individuals. Its most consistently reported mechanism? Regulation of IL-2, a master cytokine critical for T cell proliferation and immune homeostasis.
Epitalon has been shown to increase IL-2 mRNA expression in both human in vitro and animal in vivo studies, but its effects appear especially pronounced in aged tissues [4, 48], pointing toward a role in immunorejuvenation rather than broad immunostimulation. In human splenocyte cultures, IL-2 levels rose significantly within just five hours of exposure [17]. Similarly, in aged rodents, both intranasal and intramuscular administration of Epitalon led to elevated IL-2 expression specifically in certain hypothalamic nuclei [49]. This targeted, or regioselective, activation of IL-2 may help explain the broader improvements in neuroimmune integration and stress resilience observed in older biological systems.
The CD4+/CD8+ ratio serves as a key marker of immune system aging, with a shift toward CD8+ dominance often linked to chronic inflammation, reduced pathogen defense, and heightened all-cause mortality risk. In aged mice and human-derived immune cells from older individuals, Epitalon treatment was shown to increase CD4+ T cell populations within the bone marrow, helping to restore a more youthful and balanced immune profile [1]. At the same time, it preserved the homeostasis of CD8+ splenocytes, maintaining their cytotoxic function without provoking an excessive inflammatory response [50], suggesting Epitalon’s role in recalibrating, rather than overactivating, immune function.
This modulation is not a blanket immune “boost,” but a corrective rebalancing more akin to restoring the orchestration of the immune system than simply turning up the volume.
Interestingly, Epitalon’s effects appear to vary based on the biological age of the subject. In young mice, it suppressed the release of pro-inflammatory cytokines from macrophages, indicating a potential role in tempering an overactive immune response during youth [44]. In contrast, aged animals exhibited no significant changes in macrophage cytokine levels, suggesting that Epitalon’s therapeutic value may lie more in correcting age-related immune deficits than in stimulating already functional systems. This context-sensitive, age-specific modulation underscores Epitalon’s potential as a precision tool in longevity-focused interventions.
One of the more novel findings is that Epitalon doesn’t just act on peripheral immune tissues; it also influences central immune command centers, particularly the hypothalamus. Epitalon increased IL-2 expression in targeted brain regions, implying that it may recalibrate immune tone from the top down [52]. This could explain observed improvements in stress resilience, circadian rhythm, and systemic inflammatory balance across multiple models.
The immune system doesn’t age in isolation. It drives and reflects the aging of every other system. By restoring cytokine signaling, correcting T cell imbalances, and potentially reprogramming the central neuroimmune axis, Epitalon positions itself not as an immune stimulant, but as an immune homeostasis modulator.
This holds particular promise for:
- Older adults facing chronic low-grade inflammation
- Immunocompromised individuals
- Age-related decline in vaccine efficacy
- Oncology patients recovering from chemo- or radiotherapy-induced immune depletion
In the next section, we turn to the brain by examining Epitalon’s neuroprotective potential and early findings on cognitive health and neurodegeneration.
Neuroprotective Effects and Cognitive Health: What the Early Studies Say
The aging brain is a battleground. As neurons shrink, oxidative stress rises, and synaptic density fades, memory, focus, and resilience begin to erode. While no peptide can halt this entirely, early evidence suggests Epitalon may help preserve key cognitive functions by intervening at multiple points in the neurodegenerative cascade.
In both cellular and animal models, Epitalon shows promise in enhancing neuroplasticity, maintaining cholinergic tone, and reducing oxidative DNA damage, all hallmarks of both healthy brain aging and resistance to cognitive decline.
In human SH-SY5Y neuroblastoma cells, a well-established model for studying neuronal behavior, Epitalon significantly increased the secretion of soluble amyloid precursor protein (sAPP) by ~20%. This neuroprotective form of APP contrasts with the pathogenic amyloid-beta fragments that are implicated in Alzheimer’s disease. It also increased acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) activity, key enzymes that modulate acetylcholine, the neurotransmitter crucial for learning and memory [1].
These results position Epitalon as a potential candidate for stabilizing cholinergic tone, a critical early disruption in age-related cognitive decline, and for addressing cognitive disorders associated with cholinergic deficiency and amyloid metabolism.
One of the most visually striking findings (Fig. 3) came from studies in which human fibroblasts were reprogrammed into neurons, then treated with Epitalon [53]. The results were notable:
- Dendritic length increased
- Branching complexity rose
- Total junction counts went up, indicating enhanced synaptic connectivity and plasticity
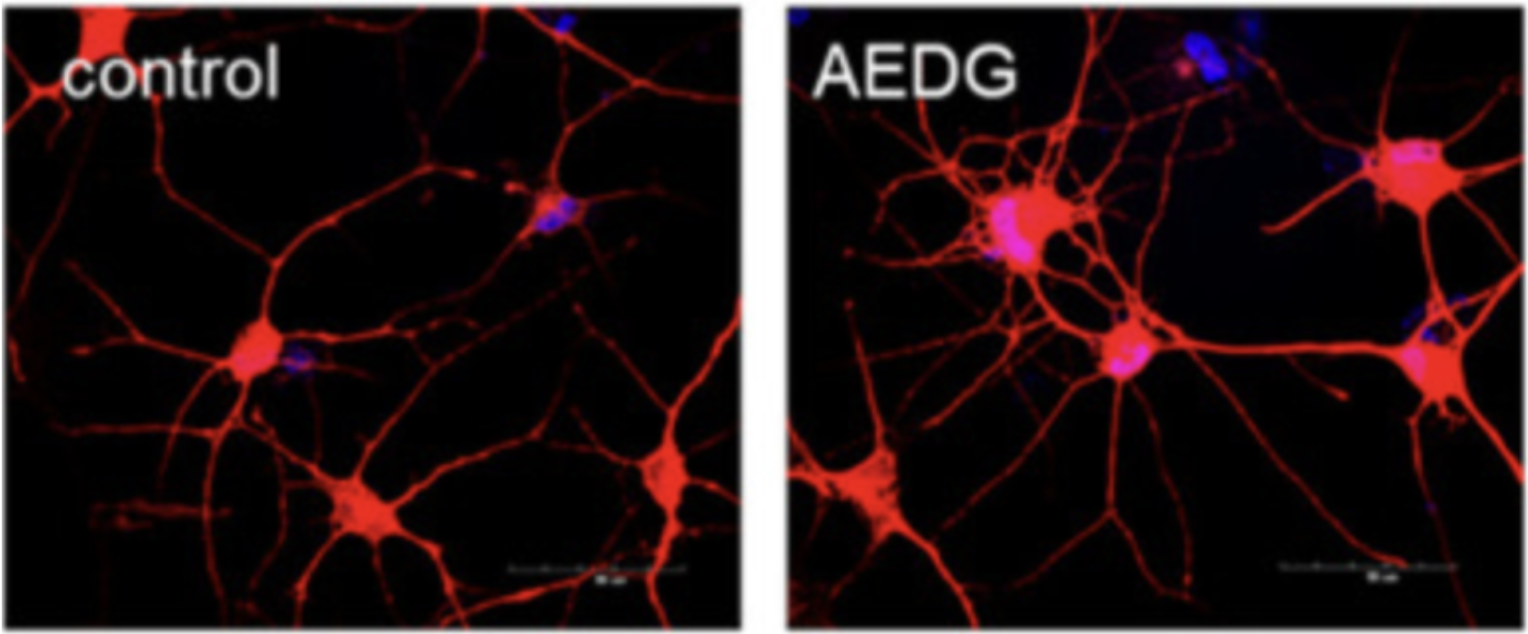
Figure 3. Morphological differences between untreated control cells and those exposed to the AEDG peptide (Epitalon). Compared with controls, Epitalon-treated cells display distinct structural changes consistent with its reported cytoprotective and regenerative effects. Image from Kraskovskaya et al. (2024).
These morphological changes are essential for preserving learning capacity, memory encoding, and neural resilience in the face of aging or injury.
Neurons are especially vulnerable to oxidative stress due to their high energy demands and limited regeneration capacity. In neuroblastoma cells, Epitalon reduced levels of 8-hydroxydeoxyguanosine (8-OHdG), a key marker of oxidative DNA damage that accumulates in aging neurons and is elevated in Alzheimer’s and Parkinson’s disease [51]. By reducing 8-OHdG, Epitalon may contribute to neuroprotection at the genomic level, preserving both structural integrity and functional longevity of neurons.
In studies using human gingival and periodontal stem cells [4, 45], Epitalon enhanced the expression of:
- Nestin (neural progenitor marker)
- GAP43 (axon growth)
- β-Tubulin III and Doublecortin (neuronal differentiation and migration)
Together, these shifts suggest Epitalon may prime stem cells toward neuronal lineage, hinting at applications in regenerative neurology or brain injury recovery models.
In a rat model of retinal degeneration, prenatal and postnatal administration of Epitalon preserved retinal structure and doubled electrophysiological retinal activity [55]. Although focused on the eye, these findings reflect broader CNS protection and raise the possibility that early intervention with Epitalon may buffer against age-related sensory decline.
Cognitive decline is not inevitable, but it is multi-factorial. What makes Epitalon interesting is that it doesn’t just tackle one element - it touches multiple levels of neuronal health:
- Neurotransmitter regulation
- Synaptic plasticity
- Oxidative DNA protection
- Stem cell priming toward neurogenesis
While human clinical data is still in its infancy, these cellular and animal results point to real potential for Epitalon in cognitive longevity strategies, neurodegenerative disease prevention, or even post-injury neural recovery.
Next, we shift from the brain to the circadian system to examine how Epitalon resets melatonin rhythms and why that matters far beyond sleep.
Epitalon and Hormonal Regulation: Resetting the Circadian Clock
Aging is more than wrinkles and fatigue. It’s a progressive desynchronization of the body’s biological rhythms. Hormonal cascades, sleep-wake cycles, and immune signals begin to drift out of sync, contributing to everything from metabolic dysfunction to neurodegeneration. One of the central players in this story is melatonin, and emerging data suggest that Epitalon may restore its natural rhythm.
By targeting melatonin synthesis and circadian gene expression, Epitalon could act as a molecular metronome, realigning internal timekeeping mechanisms that tend to falter with age.
Melatonin is synthesized in the pineal gland through the activation of two key enzymes:
- AANAT (arylalkylamine N-acetyltransferase)
- pCREB (phosphorylated cAMP response element-binding protein)
In rat pinealocytes, Epitalon was shown to upregulate both AANAT and pCREB, suggesting a direct enzymatic stimulation of melatonin biosynthesis [56].
Epitalon not only boosts melatonin production but also appears to directly protect pineal tissue itself from age-related decline. In aged human pinealocytes, Epitalon selectively safeguarded cells from degenerative changes, suggesting a tissue-specific rejuvenating effect on the gland responsible for circadian control. These findings strengthen the hypothesis that Epitalon’s chronobiotic activity extends beyond hormonal stimulation because it may also preserve the structural and cellular integrity of the pineal gland as we age [57].
These effects extend beyond in vitro experiments. In aged Rhesus monkeys, Epitalon treatment stimulated melatonin production and restored youthful secretion patterns, while also normalizing cortisol rhythms in a time-of-day–dependent manner [59]. Together, these changes suggest that Epitalon may support broader endocrine recalibration in aging organisms.
In a randomized clinical study involving 75 women, sublingual Epitalon was administered at a dose of 0.5 mg/day for 20 days, with placebo and control cohorts for comparison. Melatonin synthesis was evaluated by measuring urinary 6-sulfatoxymelatonin, the primary metabolite of melatonin, which increased 1.6-fold in the Epitalon group relative to placebo. At the genomic level, Epitalon modulated the expression of key circadian rhythm genes: Clock expression in leukocytes decreased 1.8-fold, Cry2 expression doubled, and Csnk1e expression in lymphocytes decreased by 2.1-fold, all with statistical significance. These findings indicate that Epitalon’s effects extend beyond simply boosting melatonin output, acting as a true circadian regulator capable of re-entraining disrupted biological rhythms in aging humans.
While Epitalon appears to support melatonin synthesis overall, studies also show it behaves non-linearly in pinealocyte cultures. At certain concentrations and time points, overstimulation may suppress rather than enhance melatonin output, highlighting the importance of dose, timing, and context when considering clinical protocols [1].
This reinforces the need for carefully titrated regimens, especially in clinical settings where circadian timing (chronopharmacology) may dictate efficacy.
Often dismissed as a sleep aid, melatonin is far more than a circadian signal. It plays integral roles in:
- Immune regulation
- Cancer suppression (anti-proliferative and pro-apoptotic signaling)
- Mitochondrial function and redox balance
- Hormonal feedback loops, including cortisol and estrogen/testosterone rhythms
Aging diminishes melatonin output by up to 75%, leading to weaker circadian cues and downstream systemic dysfunction. Epitalon’s capacity to restore melatonin output and genomic rhythm may therefore impact lifespan and healthspan well beyond sleep improvement. The circadian clock is a master regulator of aging biology. Disruptions in rhythm accelerate cellular aging, impair immune surveillance, and increase disease risk across all major systems of the body.
By modulating key enzymes, boosting melatonin output, and resetting clock gene expression, Epitalon may function as a chronobiotic peptide, realigning internal time to preserve physiological harmony. While more clinical trials are needed, these early findings suggest a potent role in hormonal resilience and circadian health restoration.
Next, we explore Epitalon’s anti-tumor potential, where its antimutagenic and gene-silencing effects begin to intersect with oncology.
Anti-Tumor Effects: Is Epitalon a Cancer-Preventive Peptide?
Caution: Epitalon is not a cancer treatment. Its role lies in genomic maintenance and early-stage risk reduction, not tumor eradication.
Cancer is often seen as a disease of genetic chaos driven by mutations, chromosomal instability, and uncontrolled cellular division. What makes Epitalon intriguing in the oncology space is not that it targets tumors directly, but that it appears to stabilize genomic integrity that allows cancer to flourish in the first place.
While no human clinical trials have yet tested Epitalon in cancer therapy, a growing body of animal studies suggests that it may reduce mutation load, normalize gene expression, and delay the onset of tumors, especially in aging or genetically predisposed systems.
Genomic instability is a defining hallmark of cancer. Across several preclinical models, Epitalon demonstrated a reduction in chromosomal aberrations, i.e., those misalignments, breaks, and translocations that set the stage for malignant transformation.
In SAMP mice, which naturally exhibit accelerated aging and genome instability, Epitalon treatment stabilized chromatin structure, supporting a preventative mechanism rooted in epigenetic control and DNA repair [61]
One of the most compelling cancer prevention models is the HER-2/neu transgenic mouse, engineered to spontaneously develop mammary tumors. In this model, Epitalon reduced the overall incidence of breast adenocarcinoma; tumors that did develop appeared later and were smaller in size, and the total multiplicity of tumors per mouse was significantly reduced [62]. Importantly, HER-2/neu overexpression is a clinically relevant target in human breast cancers, making this model highly translational for cancer risk mitigation in susceptible populations. Epitalon was also found to downregulate HER-2/neu gene expression, likely through modulation of chromatin structure or transcriptional regulation. Though the exact pathway is not yet fully delineated, it underscores the possibility that Epitalon influences oncogene expression, not merely downstream tumor growth.
In a chemically induced colon cancer model using 1,2-dimethylhydrazine (DMH), rats received Epitalon treatment before, during, and after carcinogen exposure. Across all time points, Epitalon consistently reduced overall tumor burden and slowed disease progression, indicating both a preventative effect and an ability to interfere with tumor promotion and advancement [63].
By modulating the epigenetic landscape, Epitalon may restore silencing of oncogenes or reactivate tumor suppressors in cells drifting toward dysplasia.
It’s essential to distinguish cancer prevention from cancer treatment. There is:
- No evidence that Epitalon can shrink existing tumors in humans
- No human clinical trials testing its use in oncology
- No mechanistic studies confirming how Epitalon affects tumor microenvironments or immune evasion in vivo
What we do have is a compelling preventive profile in animal models, especially where aging and genetic susceptibility intersect.
Cancer is not an event; it’s a process. The earlier we can preserve genome integrity, moderate oncogene expression, and prevent accumulation of damage, the lower the lifetime cancer risk. Epitalon may not be a cancer drug, but it could be part of a future preventive protocol: a peptide that helps keep the cellular script clean before the chaos begins.
Our final section will bring these threads together: Is Epitalon ready for prime time?
Final Verdict: Does Epitalon Live Up to the Hype?
Epitalon is mechanistically robust, translationally immature, but positioned as a serious contender in future longevity protocols.
Epitalon stands as one of the most extensively studied synthetic peptides in the longevity science canon, and for good reason. Over the past two decades, it has consistently demonstrated multimodal geroprotective effects across cellular, animal, and early human studies. From telomere maintenance to melatonin regulation, antioxidant defenses, and even tumor suppression, Epitalon presents a compelling narrative backed by molecular evidence.
But like many promising interventions in the longevity space, the question is not just can it work?, but can it work in humans, safely and reliably?
Across preclinical studies, Epitalon has shown:
- Telomerase activation and telomere extension: Via TRAP assays and fibroblast lifespan extension beyond the Hayflick limit
- Epigenetic remodeling: Reversal of chromatin aging, histone modulation, and gene promoter binding
- Redox homeostasis: Increased antioxidant enzymes (SOD, NQO1, catalase) and reduced ROS, lipid peroxidation, and DNA damage
- Circadian restoration: Upregulation of AANAT/pCREB, normalized melatonin and cortisol rhythms, and clock gene modulation in humans
- Cancer risk reduction: Reduced tumor multiplicity and delayed onset in HER-2/neu and colon cancer models; antimutagenic effects in lymphocytes
Each of these domains plays a foundational role in biological aging, and Epitalon’s ability to modulate them suggests its legitimate potential as a systemic geroprotector.
Despite this promise, several critical limitations remain:
- No large-scale, randomized controlled human trials to confirm efficacy, safety, or long-term impact
- Toxicology and pharmacokinetic data are lacking, especially for chronic use or high-dose regimens
- Only one stereoisomer (all-L form) has been tested; the remaining seven forms are uncharacterized in humans
- Bioavailability remains suboptimal, with oral administration largely ineffective due to enzymatic degradation; as a result, subcutaneous injections and intranasal delivery are the predominant methods used in research, though both routes present practical challenges for routine clinical use.
- Regulatory uncertainty limits its medical positioning, currently placing it in the grey zone of experimental peptides
These limitations make clinical endorsement premature, especially in regulated health systems or for medical-grade use.
To move Epitalon from experimental to evidence-based, several developments are essential:
- Randomized, double-blind, placebo-controlled clinical trials, ideally targeting specific endpoints (sleep, immune aging, telomere attrition)
- Toxicology and long-term safety studies, including exploration of stereoisomer effects and off-target consequences
- Validated delivery systems (e.g., dendrimer-conjugates, transdermal patches) that make real-world use feasible
- Pharmacodynamic biomarkers, to identify responders and track efficacy objectively over time
Epitalon is not snake oil, but it’s also not a magic bullet.
It represents a scientifically grounded, mechanistically plausible peptide with significant translational promise. For scientists and clinicians, it’s an excellent candidate for further study. For biohackers, it’s a peptide worth watching, but with an informed eye on risk, variability, and lack of regulatory oversight. For wellness professionals, it may eventually become part of a broader longevity toolkit, pending proper validation.
In short, Epitalon lives up to its hype mechanistically, but falls short in translational readiness. It is not ready for mainstream adoption - yet.
But with the right trials, safety profiling, and delivery innovation, it just might be.
In the meantime, other well-studied protocols already exist to target these same hallmarks of aging, ranging from pharmacological approaches like rapamycin and SGLT-2 inhibitors to lifestyle strategies such as exercise and caloric restriction. These options underscore that while Epitalon remains experimental, practical interventions for extending healthspan are available today.
- Araj SK, Brzezik J, Mądra-Gackowska K, Szeleszczuk Ł. Overview of Epitalon-Highly Bioactive Pineal Tetrapeptide with Promising Properties. Int J Mol Sci 2025;26:2691. https://doi.org/10.3390/ijms26062691
- Implications of NAD+ boosters in translational medicine - Kang - 2020 - European Journal of Clinical Investigation - Wiley Online Library n.d. (accessed September 18, 2025).
- Cellular senescence and senolytics: the path to the clinic | Nature Medicine n.d. https://www.nature.com/articles/s41591-022-01923-y (accessed September 18, 2025).
- Khavinson V, Diomede F, Mironova E, Linkova N, Trofimova S, Trubiani O, et al. AEDG Peptide (Epitalon) Stimulates Gene Expression and Protein Synthesis during Neurogenesis: Possible Epigenetic Mechanism. Molecules 2020;25:609. https://doi.org/10.3390/molecules25030609
- Ilahi S, Beriwal N, Ilahi TB. Physiology, Pineal Gland. StatPearls, Treasure Island (FL): StatPearls Publishing; 2025.
- Samanta S. Physiological and pharmacological perspectives of melatonin. Arch Physiol Biochem 2022;128:1346–67. https://doi.org/10.1080/13813455.2020.1770799
- Khavinson VKh, Kopylov AT, Vaskovsky BV, Ryzhak GA, Lin’kova NS. Identification of Peptide AEDG in the Polypeptide Complex of the Pineal Gland. Bull Exp Biol Med 2017;164:41–3. https://doi.org/10.1007/s10517-017-3922-8
- Kozina LS, Arutjunyan AV, Khavinson VKh. Antioxidant properties of geroprotective peptides of the pineal gland. Arch Gerontol Geriatr 2007;44:213–6. https://doi.org/10.1016/j.archger.2007.01.029
- Khavinson VKh, Konovalov SS, Yuzhakov VV, Popuchiev VV, Kvetnoi IM. Modulating Effects of Epithalamin and Epithalon on the Functional Morphology of the Spleen in Old Pinealectomized Rats. Bull Exp Biol Med 2001;132:1116–20. https://doi.org/10.1023/A:1017989113287
- Tang W, Black AS, Moench R, Marzban K, Garay JAR, Zheng JJ, et al. Trifluoroacetate reduces plasma lipid levels and the development of atherosclerosis in mice 2025:2025.03.06.641713. https://doi.org/10.1101/2025.03.06.641713
- Khavinson VK, Egorova VV, Timofeeva NM, Malinin VV, Gordova LA, Gromova LV. Effect of Vilon and Epithalon on glucose and glycine absorption in various regions of small intestine in aged rats. Bull Exp Biol Med 2002;133:494–6. https://doi.org/10.1023/a:1019878224754
- Al-dulaimi S, Thomas R, Matta S, Roberts T. Epitalon increases telomere length in human cell lines through telomerase upregulation or ALT activity 2025. https://doi.org/10.21203/rs.3.rs-7066545/v1
- Khavinson VK, Bondarev IE, Butyugov AA. Epithalon peptide induces telomerase activity and telomere elongation in human somatic cells. Bull Exp Biol Med 2003;135:590–2. https://doi.org/10.1023/a:1025493705728
- Khavinson VKh, Pendina AA, Efimova OA, Tikhonov AV, Koltsova AS, Krapivin MI, et al. Effect of Peptide AEDG on Telomere Length and Mitotic Index of PHA-Stimulated Human Blood Lymphocytes. Bull Exp Biol Med 2019;168:141–4. https://doi.org/10.1007/s10517-019-04664-0
- Ullah S, Haider Z, Perera CD, Lee SH, Idrees M, Park S, et al. Epitalon-activated telomerase enhance bovine oocyte maturation rate and post-thawed embryo development. Life Sci 2025;362:123381. https://doi.org/10.1016/j.lfs.2025.123381
- Gatta M, Dovizio M, Milillo C, Ruggieri AG, Sallese M, Antonucci I, et al. The Antioxidant Tetrapeptide Epitalon Enhances Delayed Wound Healing in an in Vitro Model of Diabetic Retinopathy. Stem Cell Rev Rep 2025;21:1822–34. https://doi.org/10.1007/s12015-025-10911-x
- Kazakova TB, Barabanova SV, Khavinson VKh, Glushikhina MS, Parkhomenko EP, Malinin VV, et al. In Vitro Effect of Short Peptides on Expression of Interleukin-2 Gene in Splenocytes. Bull Exp Biol Med 2002;133:614–6. https://doi.org/10.1023/A:1020210615148
- Linkova NS, Khavinson VKh, Chalisova NI, Katanugina AS, Koncevaya EA. Peptidegic Stimulation of Differentiation of Pineal Immune Cells. Bull Exp Biol Med 2011;152:124–7. https://doi.org/10.1007/s10517-011-1470-1
- Zhou L, Bryant CD, Loudon A, Palmer AA, Vitaterna MH, Turek FW. The Circadian Clock Gene Csnk1e Regulates Rapid Eye Movement Sleep Amount, and Nonrapid Eye Movement Sleep Architecture in Mice. Sleep 2014;37:785–93. https://doi.org/10.5665/sleep.3590
- Ilina A, Khavinson V, Linkova N, Petukhov M. Neuroepigenetic Mechanisms of Action of Ultrashort Peptides in Alzheimer’s Disease. Int J Mol Sci 2022;23:4259. https://doi.org/10.3390/ijms23084259
- Lee J, Pellegrini MV. Biochemistry, Telomere And Telomerase. StatPearls, Treasure Island (FL): StatPearls Publishing; 2025.
- The relationship between telomere length and aging-related diseases | Clinical and Experimental Medicine n.d. https://link.springer.com/article/10.1007/s10238-025-01608-z (accessed September 18, 2025).
- Liu Y, Schwam J, Chen Q. Senescence-Associated Cell Transition and Interaction (SACTAI): A Proposed Mechanism for Tissue Aging, Repair, and Degeneration. Cells 2022;11:1089. https://doi.org/10.3390/cells11071089
- Lee J, Pellegrini MV. Biochemistry, Telomere And Telomerase. StatPearls, Treasure Island (FL): StatPearls Publishing; 2025.
- Khavinson VKh, Bondarev IE, Butyugov AA. Epithalon Peptide Induces Telomerase Activity and Telomere Elongation in Human Somatic Cells. Bull Exp Biol Med 2003;135:590–2.
- Peptide Promotes Overcoming of the Division Limit in Human Somatic Cell | Bulletin of Experimental Biology and Medicine n.d. https://link.springer.com/article/10.1023/B:BEBM.0000038164.49947.8c (accessed September 18, 2025).
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, et al. Substrate-specific Activation of Sirtuins by Resveratrol*. J Biol Chem 2005;280:17038–45. https://doi.org/10.1074/jbc.M500655200
- Huang X, Huang L, Lu J, Cheng L, Wu D, Li L, et al. The relationship between telomere length and aging-related diseases. Clin Exp Med 2025;25:72. https://doi.org/10.1007/s10238-025-01608-z
- Jinesh S, Özüpek B, Aditi P. Premature aging and metabolic diseases: the impact of telomere attrition. Front Aging 2025;6. https://doi.org/10.3389/fragi.2025.1541127
- Rossiello F, Jurk D, Passos JF, d’Adda di Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol 2022;24:135–47. https://doi.org/10.1038/s41556-022-00842-x
- Tiffon C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int J Mol Sci 2018;19:3425. https://doi.org/10.3390/ijms19113425
- Fedoreyeva LI, Kireev II, Khavinson VKh, Vanyushin BF. Penetration of short fluorescence-labeled peptides into the nucleus in HeLa cells and in vitro specific interaction of the peptides with deoxyribooligonucleotides and DNA. Biochem Mosc 2011;76:1210–9. https://doi.org/10.1134/S0006297911110022
- Attina G, Mastrangelo S, Ruggiero A. Telomerase and Anticancer Treatment. Biomed Pharmacol J 2022;15:1881–8.
- Lee J-H, Kim EW, Croteau DL, Bohr VA. Heterochromatin: an epigenetic point of view in aging. Exp Mol Med 2020;52:1466–74. https://doi.org/10.1038/s12276-020-00497-4
- Khavinson VK, Lezhava TA, Monaselidze JR, Jokhadze TA, Dvalishvili NA, Bablishvili NK, et al. Peptide Epitalon activates chromatin at the old age. Neuro Endocrinol Lett 2003;24:329–33
- Epigenetic Variations in Chromatin Caused by the Combination of Bioregulators with Heavy Metals During Aging | International Journal of Peptide Research and Therapeutics n.d. https://link.springer.com/article/10.1007/s10989-022-10427-9 (accessed September 18, 2025).
- Solovyev AY, Tarnovskaya SI, Chernova IA, Shataeva LK, Skorik YA. The interaction of amino acids, peptides, and proteins with DNA. Int J Biol Macromol 2015;78:39–45. https://doi.org/10.1016/j.ijbiomac.2015.03.054
- Aging Hallmarks and the Role of Oxidative Stress n.d. https://www.mdpi.com/2076-3921/12/3/651 (accessed September 19, 2025).
- Giorgi C, Marchi S, Simoes ICM, Ren Z, Morciano G, Perrone M, et al. Chapter Six - Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. In: López-Otín C, Galluzzi L, editors. Int. Rev. Cell Mol. Biol., vol. 340, Academic Press; 2018, p. 209–344. https://doi.org/10.1016/bs.ircmb.2018.05.006
- Short peptides stimulate cell regeneration in skin during aging | Advances in Gerontology n.d. https://link.springer.com/article/10.1134/S2079057015030054 (accessed September 19, 2025).
- Gutop EO, Linkova NS, Kozhevnikova EO, Fridman NV, Ivko OM, Khavinson VKh. AEDG Peptide Prevents Oxidative Stress in the Model of Induced Aging of Skin Fibroblasts. Adv Gerontol 2022;12:143–8. https://doi.org/10.1134/S2079057022020096
- Short Peptides Protect Oral Stem Cells from Ageing | Stem Cell Reviews and Reports n.d. https://link.springer.com/article/10.1007/s12015-019-09921-3 (accessed September 19, 2025).
- Khavinson VKh, Yuzhakov VV, Kvetnoi IM, Malinin VV, Popuchiev VV, Fomina NK. Immunohistochemical and Morphometric Analysis of Effects of Vilon and Epithalon on Functional Morphology of Radiosensitive Organs. Bull Exp Biol Med 2001;131:285–92. https://doi.org/10.1023/A:1017676104877
- Khavinson VKh, Izmaylov DM, Obukhova LK, Malinin VV. Effect of epitalon on the lifespan increase in Drosophila melanogaster. Mech Ageing Dev 2000;120:141–9. https://doi.org/10.1016/S0047-6374(00)00217-7
- Anisimov VN, Khavinson VKh, Mikhalski AI, Yashin AI. Effect of synthetic thymic and pineal peptides on biomarkers of ageing, survival and spontaneous tumour incidence in female CBA mice. Mech Ageing Dev 2001;122:41–68. https://doi.org/10.1016/S0047-6374(00)00184-6
- Epitalon protects against post-ovulatory aging-related damage of mouse oocytes in vitro | Aging n.d. https://www.aging-us.com/article/204007/text (accessed September 19, 2025)
- Balamurugan BS, Marimuthu MMC, Sundaram VA, Saravanan B, Chandrababu P, Chopra H, et al. Micro nutrients as immunomodulators in the ageing population: a focus on inflammation and autoimmunity. Immun Ageing 2024;21:88. https://doi.org/10.1186/s12979-024-00492-7
- Gumen AV, Kozinets IA, Shanin SN, Malinin VV, Rybakina EG. Production of lymphocyte-activating factors by mouse macrophages during aging and under the effect of short peptides. Bull Exp Biol Med 2006;142:360–2. https://doi.org/10.1007/s10517-006-0366-y
- Kazakova TB, Barabanova SV, Novikova NS, Glushikhina MS, Khavinson VKh, Malinin VV, et al. Synthesis of IL-2 mRNA in Cells of Rat Hypothalamic Structures after Injection of Short Peptides. Bull Exp Biol Med 2005;139:718–20. https://doi.org/10.1007/s10517-005-0388-x
- Labunets IF, Butenko GM, Khavinson VK. Effects of bioactive factors of the pineal gland on thymus function and cell composition of the bone marrow and spleen in mice of different age. Bull Exp Biol Med 2004;137:510–2. https://doi.org/10.1023/b:bebm.0000038166.54823.06
- Korenevsky AV, Milyutina YP, Bukalyov AV, Baranova YP, Vinogradova IA, Arutjunyan AV. [Protective effect of melatonin and epithalon on hypothalamic regulation of reproduction in female rats in its premature aging model and on estrous cycles in senescent animals in various lighting regimes]. Adv Gerontol Uspekhi Gerontol 2013;26:263–74.
- Barabanova SV, Artyukhina ZE, Kazakova TB, Khavinson VK, Malinin VV, Korneva EA. Interleukin-2 concentration in hypothalamic structures of rats receiving peptides during mild stress. Bull Exp Biol Med 2006;141:390–3. https://doi.org/10.1007/s10517-006-0179-z
- Kraskovskaya N, Linkova N, Sakhenberg E, Krieger D, Polyakova V, Medvedev D, et al. Short Peptides Protect Fibroblast-Derived Induced Neurons from Age-Related Changes. Int J Mol Sci 2024;25:11363. https://doi.org/10.3390/ijms252111363
- Caputi S, Trubiani O, Sinjari B, Trofimova S, Diomede F, Linkova N, et al. Effect of short peptides on neuronal differentiation of stem cells. Int J Immunopathol Pharmacol 2019;33:2058738419828613. https://doi.org/10.1177/2058738419828613
- Khavinson VK, Zemchikhina VN, Trofimova SV, Malinin VV. Effects of peptides on proliferative activity of retinal and pigmented epithelial cells. Bull Exp Biol Med 2003;135:597–9. https://doi.org/10.1023/a:1025497806636
- Khavinson VK, Linkova NS, Kvetnoy IM, Kvetnaia TV, Polyakova VO, Korf H-W. Molecular cellular mechanisms of peptide regulation of melatonin synthesis in pinealocyte culture. Bull Exp Biol Med 2012;153:255–8. https://doi.org/10.1007/s10517-012-1689-5
- Ivko OM, Drobintseva AO, Leont’eva DO, Kvetnoy IM, Polyakova VO, Linkova NS. Influence of AEDG and KE Peptides on Mitochondrial Staining and the Expression of Ribosomal Protein L7A with Aging of the Human Pineal Gland and Thymus Cell In Vitro. Adv Gerontol 2021;11:261–7. https://doi.org/10.1134/S2079057021030061
- Khavinson V, Goncharova N, Lapin B. Synthetic tetrapeptide epitalon restores disturbed neuroendocrine regulation in senescent monkeys. Neuro Endocrinol Lett 2001;22:251–4.
- Goncharova ND, Khavinson BK, Lapin BA. Regulatory effect of Epithalon on production of melatonin and cortisol in old monkeys. Bull Exp Biol Med 2001;131:394–6. https://doi.org/10.1023/a:1017928925177
- Rubinskii AV, Linkova NS, Chalisova NI, Noskin LA, Marchenko VN, Khavinson VK. [Epigenetic regulation of adaptogenesis by pathology and aging. Adv Gerontol Uspekhi Gerontol 2021;34:10–7.
- Rosenfeld SV, Togo EF, Mikheev VS, Popovich IG, Khavinson VK, Anisimov VN. Effect of epithalon on the incidence of chromosome aberrations in senescence-accelerated mice. Bull Exp Biol Med 2002;133:274–6. https://doi.org/10.1023/a:1015899003974.
- Anisimov VN, Khavinson VKH, Provinciali M, Alimova IN, Baturin DA, Popovich IG, et al. Inhibitory effect of the peptide epitalon on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int J Cancer 2002;101:7–10. https://doi.org/10.1002/ijc.10570
- Anisimov VN, Khavinson VKh, Popovich IG, Zabezhinski MA. Inhibitory effect of peptide Epitalon on colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Cancer Lett 2002;183:1–8.
- Khavinson VK, Razumovsky MI, Trofimova SV, Razumovskaya AM. Retinoprotective effect of Epithalon in campbell rats of various ages. Bull Exp Biol Med 2003;135:495–8.
- Khavinson VK, Razumovskii MI, Trofimova SV, Grigor’yan RA, Chaban TV, Oleinik TL, et al. Effect of epithalon on age-specific changes in the retina in rats with hereditary pigmentary dystrophy. Bull Exp Biol Med 2002;133:87–9. https://doi.org/10.1023/a:1015125031829
- Fatullaev EI, V.V.Bezrodnyi, Neelov IM. MD Simulation of AEDG Peptide Complexes with New K2R Dendrimer and Dendrigraft. Int J Biol Biomed Eng 2022;16:73–81. https://doi.org/10.46300/91011.2022.16.9
Related studies