A Multi-System Shift: How SGLT2 Inhibitors Target the Hallmarks of Aging—Telomeres, Immune Function, and Metabolic Reprogramming
Prefer to listen? Hit play for a conversational, audio‑style summary of this article’s key points.
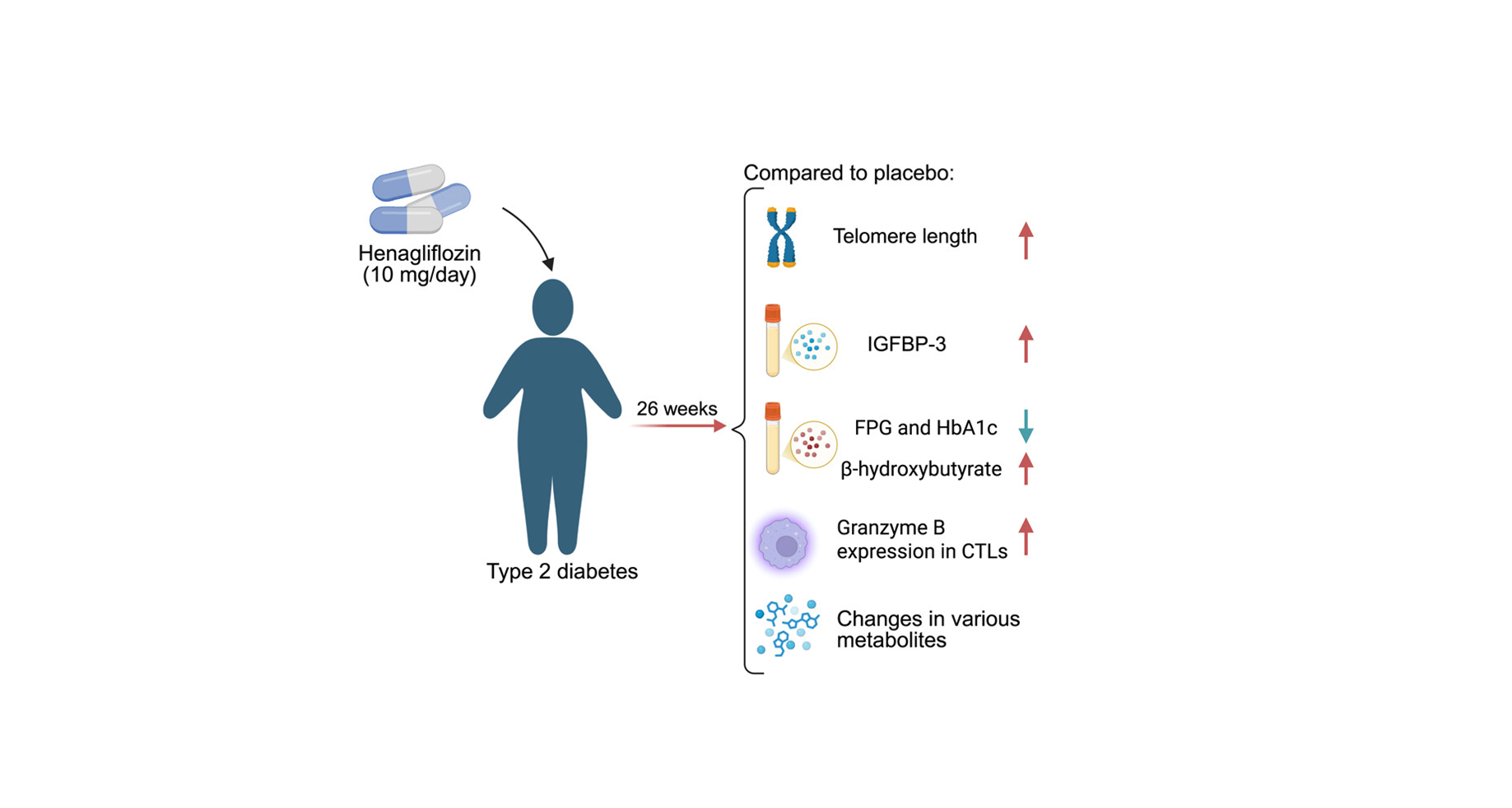
First randomized human trial to test an SGLT2 inhibitor on core aging biology: Henagliflozin is the first SGLT2 inhibitor studied in a placebo-controlled design with aging biomarkers—telomeres, IGF-1 signaling, immune-cell function, and metabolomics—showing coordinated improvements across multiple hallmarks of aging.
Telomere length increased significantly over 26 weeks: Participants receiving henagliflozin had a statistically significant rise in leukocyte telomere length, with 90.5% showing telomere lengthening versus 65.6% in placebo—an unusually rapid shift for a metric that typically changes slowly.
IGFBP-3 rose, nudging the GH/IGF-1 axis toward a longevity-associated state: Henagliflozin increased IGFBP-3 (p = 0.013), suggesting reduced IGF-1 exposure and a shift toward metabolic preservation rather than anabolic growth—a pattern linked with longer lifespan in many models.
Cytotoxic T-cell activity improved in ways consistent with enhanced senescent-cell clearance: Granzyme B, a key enzyme CTLs use to destroy senescent and damaged cells, increased significantly in the henagliflozin group (p = 0.033). These shifts indicate stronger immune-mediated clearance pathways that normally weaken with age. Crucially, inflammatory cytokines (IL-6, IL-10, IFN-γ) did not increase, suggesting a restoration of targeted immune surveillance against senescent cells rather than broad immune activation.
β-Hydroxybutyrate rose, signaling a fasting-like metabolic transition: Henagliflozin increased β-hydroxybutyrate from 0.07 → 0.10 mmol/L (p = 0.002), reflecting a shift toward fat oxidation, cleaner mitochondrial fuel use, reduced inflammatory signaling, and activation of stress-response pathways involved in longevity.
Metabolomics revealed improved mitochondrial support and lower metabolic stress: Across 53 significantly changed metabolites, henagliflozin increased thiamine (a key mitochondrial cofactor) and decreased stress-related lipids like PC, PE, and sphingosine, indicating a calmer, lower-stress biochemical environment.
The pattern resembles caloric restriction, without dietary restriction: The combined BHB rise, IGF-1 axis modulation, reduced inflammatory lipids, and enhanced mitochondrial cofactors produced a profile strikingly similar to caloric restriction biology, long known to extend lifespan in experimental models.
Why SGLT2 Inhibitors Are Emerging as Surprising Anti-Aging Candidates
For nearly a decade, SGLT2 inhibitors such as empagliflozin, dapagliflozin, bexagliflozin, and the newer henagliflozin have been reshaping the treatment of type 2 diabetes. These medications were originally designed to lower blood glucose by helping the kidneys remove excess sugar from the blood in urine. Soon after their introduction, it became clear that their impact reached far beyond glucose control. Clinical trials consistently showed benefits across three major systems: metabolic regulation, cardiovascular protection, and kidney health, all within a safety profile that has made them widely used and well-trusted.
The same features that make these drugs effective in metabolic disease have drawn growing attention from researchers studying human longevity. SGLT2 inhibitors reduce the body’s reliance on glucose, shift cells toward burning fats, support more efficient mitochondrial energy production, and lower chronic inflammation. Many of these are the same physiological changes observed during caloric restriction, an intervention known to extend lifespan in several species. Because of this overlap, SGLT2 inhibitors are now being investigated as potential “caloric-restriction mimetics,” capable of producing fasting-like cellular benefits without major dietary changes.
Despite this interest, most discussions about SGLT2 inhibitors and aging still rely on routine clinical measurements: blood sugar, body weight, and kidney filtration rate. What has been missing is evidence on deeper biological markers, including the molecular processes that influence cellular aging. One especially important marker is telomere length. Telomeres are repeating DNA sequences that cap the ends of chromosomes, protecting genetic material during cell division. As telomeres shorten over time, cells become less capable of repair and more prone to senescence. Whether a common metabolic drug could slow or reverse this erosion had never been tested in a randomized human trial.
In recent months, a team led by Jie Zhang and Jixiong Xu published a new clinical trial in Cell Reports Medicine that marks the first randomized human test of an SGLT2 inhibitor on biological aging itself. The researchers examined whether six months of daily henagliflozin could alter several core indicators of cellular aging in adults with type 2 diabetes. Their analysis went far beyond routine glucose measurements and included telomere length, growth-factor signaling, immune-cell function, and metabolomic patterns that reveal how cells generate and use energy. The study represents an early but essential step toward understanding whether a widely used metabolic drug can reach down to the molecular machinery that governs how humans age.
Inside the First Human Trial Testing an SGLT2 Inhibitor on Biological Aging
To explore whether an SGLT2 inhibitor could influence the deeper biology of aging, not just glucose levels, Zhang and colleagues conducted a multicenter, randomized, double-blind, placebo-controlled trial across 18 hospitals in China. The drug they tested, henagliflozin, works the same way as familiar SGLT2 inhibitors such as empagliflozin, dapagliflozin, and bexagliflozin: it blocks a transporter in the kidney that usually reabsorbs glucose, leading to more sugar being excreted in the urine. This shift nudges the body toward using fat as fuel and alters several energy-sensing pathways closely tied to aging. [2]
The researchers enrolled 150 adults with type 2 diabetes who met strict inclusion criteria designed to reduce metabolic variability. Participants were 35 to 70 years old, had moderately elevated HbA1c levels (7.0 to 9.5 percent), a body mass index of at least 22 kg/m², and had not taken glucose-lowering medications for at least twelve weeks. After 26 weeks of treatment, 142 volunteers completed all primary assessments. [1]
Each participant was randomly assigned to receive either 10 mg of henagliflozin daily or an identical placebo tablet. To avoid confounding factors, everyone received standardized guidance on diet, exercise, and home glucose monitoring. Clinic visits occurred at baseline and at weeks 4, 12, and 26, followed by a safety check two weeks after the study’s end. [1]
A key feature of the trial was its selection of telomere length as the primary endpoint—an unusual and ambitious target for a metabolic drug study. Telomeres are repeating DNA sequences located at the ends of chromosomes. Their role is to act as protective caps that prevent genetic material from unraveling or fusing with other chromosomes during cell division. Every cell division and every exposure to stress, such as high blood glucose, oxidative damage, or inflammation, causes telomeres to erode slightly. When they become critically low, the cell can no longer divide and instead enters senescence, a state in which it secretes inflammatory molecules and loses its ability to repair tissues. Because of this, telomere shortening is considered a fundamental hallmark of aging. [3, 4]
In metabolic diseases like type 2 diabetes, telomere erosion accelerates. Chronically elevated glucose levels increase reactive oxygen species production, which can damage DNA, while low-grade inflammation drives faster cell turnover. As a result, people with diabetes often have shorter telomeres in their leukocytes (white blood cells), and these shorter telomeres are associated with higher risks of cardiovascular disease, neuropathy, and mortality. Preserving telomere length in this context is seen as a sign of improved cellular resilience and slower biological aging. [3]
The team quantified telomere length using quantitative polymerase chain reaction (qPCR). This well-established technique compares the amount of telomeric DNA to the amount of a stable, single-copy reference gene. This ratio, called the T/S ratio, provides an index of relative telomere length. Measurements were taken at the start of the trial and again at week 26 to determine how telomere dynamics changed with treatment. [1]
Around this central measure of genomic stability, the study also incorporated several additional biomarkers that map onto other hallmarks of aging, including IGF-1, β-hydroxybutyrate (BHB), and immune proteins. Together, these endpoints provided a layered, systems-level view of how SGLT2 inhibition might influence genomic integrity, energy metabolism, mitochondrial activity, and immune function. In other words, the study was designed not simply to monitor glucose levels, but to determine whether a common metabolic drug could shift the deeper biology that governs how human cells age. [1]
What They Found: Telomere Outcomes
The trial’s primary objective was to determine whether an SGLT2 inhibitor could alter telomere dynamics over a relatively short, 26-week period. The results were clear: henagliflozin produced a measurable increase in leukocyte telomere length compared with placebo, making this one of the first randomized, placebo-controlled human studies to show that a metabolic drug can influence a chromosomal marker of biological aging. [1]

Figure 1. Telomere Length Changes After 26 Weeks of Treatment. This figure shows how leukocyte telomere length shifted from baseline to week 26 in participants receiving henagliflozin versus placebo. [1]
However, it is important to clarify what this measurement represents. The study used qPCR to derive a T/S ratio—a relative measure of telomeric DNA compared with a single-copy gene. This method is widely used in aging research, but it does not provide an absolute base-pair estimate of telomere length. Instead, it detects proportional changes over time. Framing the results this way helps calibrate expectations: a 0.06 between-group difference may appear numerically small but is biologically meaningful for a relative assay that typically changes slowly over years.
Another notable detail is that the placebo group also experienced a modest telomere increase. The authors highlight that all participants received structured lifestyle counseling—including dietary guidance, exercise recommendations, sleep hygiene support, and glucose-monitoring education. Prior work has shown that lifestyle improvement alone can extend telomeres modestly over months (as described in the study’s reference to Buttet et al., 2022). Because both groups received identical behavioral guidance, the greater telomere gain in the henagliflozin group reflects a pharmacological effect above and beyond lifestyle changes.
Quantitatively, the mean between-group difference in telomere change was +0.06. When the data were analyzed categorically, the pattern became even clearer: 90.5% of participants taking henagliflozin showed telomere lengthening versus 65.6% on placebo. The effect remained significant after adjusting for smoking, an established telomere-accelerating factor. [1]
Taken together, these findings suggest that SGLT2 inhibition may influence telomere biology, even within a timeframe as short as six months. The magnitude of change is modest—as expected for telomere dynamics—but directionally consistent, statistically robust, and mechanistically aligned with the drug’s caloric-restriction-like metabolic shifts.
Why IGFBP-3 Matters for Longevity Biology
Beyond telomeres, one of the most compelling findings in the study involved the growth hormone/insulin-like growth factor-1 (GH/IGF-1) axis, a pathway deeply conserved across species in its influence on aging. In model organisms, reduced IGF-1 signaling is associated with slower aging, improved cellular maintenance, and extended lifespan.
A key regulator of this pathway is insulin-like growth factor binding protein-3 (IGFBP-3). Rather than serving solely as a transport protein, IGFBP-3 reduces IGF-1 bioavailability and modulates downstream signaling. In human studies, higher IGFBP-3 levels predict lower all-cause mortality, while a higher IGF-1/IGFBP-3 molar ratio predicts earlier morbidity.
In this trial, henagliflozin significantly increased IGFBP-3 levels compared with placebo (p = 0.013). Participants receiving placebo experienced a decline in IGFBP-3 (−0.17 µg/mL), whereas those on henagliflozin maintained stable levels (+0.01 µg/mL). IGF-1 levels rose modestly in both groups (+17.67 ng/mL placebo vs. +15.14 ng/mL henagliflozin, p = 0.631), and the IGF-1/IGFBP-3 molar ratio increased slightly in both arms (+0.02 vs. +0.02; p = 0.266), though these latter changes were not statistically significant.
The directionality of these shifts remains important: higher IGFBP-3, slightly lower IGF-1 exposure, and a stable molar ratio together reflect a metabolic state characterized by lower growth signaling and greater maintenance activity. Given that SGLT2 inhibitors induce a fasting-like energy profile—enhanced fat oxidation, increased β-hydroxybutyrate, and reduced glucose flux—these GH/IGF-1 axis changes fit within a coherent biological framework. Combined with the telomere findings, they suggest that henagliflozin may be nudging cellular physiology toward a lower-IGF, higher-resilience state, a pattern broadly associated with improved longevity in experimental models and population studies.
How Henagliflozin Boosted Immune Surveillance in Aging Cells
In addition to evaluating chromosomal aging, the researchers investigated whether SGLT2 inhibition could influence immunosenescence, the gradual weakening of the immune system that accompanies aging. One of the clearest manifestations of immunosenescence is the decline in the effectiveness of cytotoxic T lymphocytes (CTLs), the immune cells responsible for identifying and eliminating infected, damaged, or senescent cells. When CTLs lose functional potency, harmful or dysfunctional cells accumulate, contributing to chronic inflammation and age-related tissue decline.
To assess this domain of aging biology, the researchers conducted a targeted immune analysis in a subset of participants (19 in the henagliflozin group and 15 in the placebo group). After 26 weeks, CTLs from those receiving henagliflozin showed stronger effector signatures, meaning their molecular machinery for killing abnormal cells was more active. [1]
The clearest signal came from granzyme B, an enzyme CTLs use to trigger cell death in compromised cells. Granzyme B levels increased significantly in the henagliflozin group compared with placebo (p = 0.033). Perforin, another essential protein, also trended upward. When the researchers expanded their analysis to include total T-cell populations, both granzyme B and perforin showed similar upward trends, suggesting that the immune enhancement was not restricted to a single T-cell subtype but reflected a broader boost. [1]
Crucially, these gains occurred without accompanying rises in systemic inflammation. Circulating levels of key cytokines, including IL-6, IL-10, and IFN-γ, remained steady in both treatment groups. This distinction is important: elevated cytokines would indicate generalized immune activation, which can be harmful. Instead, the pattern observed here points to improved immune surveillance, stronger CTL function without inflammatory collateral damage. [1]
Why This Matters for Aging Biology
These findings are mechanistically aligned with emerging work in animal models. A 2024 Nature Aging study demonstrated that SGLT2 inhibition can directly clear senescent cells and reduce pathological aging in multiple tissues. In senescence biology, CTL granzyme B–perforin activity is a linchpin: senescent cells resist apoptosis, but re-empowered CTLs can overcome this resistance and restore tissue homeostasis.
Viewed through this lens, the immune enhancements in this human trial suggest that henagliflozin may counteract components of age-related immune decline by:
- Reducing metabolic and oxidative stress,
- Supporting healthier energy utilization within immune cells,
- Enhancing CTL cytotoxic capacity,
- Potentially promoting the clearance of senescent or dysfunctional cells.
This is particularly notable given that adults with type 2 diabetes often exhibit accelerated immunosenescence. Observing improvements in a population starting from this disadvantage strengthens the plausibility that these immune effects are meaningful and not merely noise.
Together, the immune data, telomere changes, and IGF-1 axis shifts all point in the same direction: henagliflozin appears to nudge the immune system toward a more youth-like functional state—one better equipped to maintain tissue integrity and eliminate unhealthy cells—without triggering unwanted inflammatory activation.
A Shift Toward Ketone-Based Metabolism: What Rising β-Hydroxybutyrate Signals in This Trial
One of the most consistent biochemical shifts observed in the study was an increase in β-hydroxybutyrate (BHB), the primary circulating ketone body produced during fasting, caloric restriction, and sustained fat oxidation. BHB has received growing attention in aging research because it functions not only as an energy substrate but also as a signaling molecule that influences inflammation, oxidative stress, mitochondrial efficiency, and cellular longevity pathways.
In the trial, henagliflozin significantly increased serum BHB levels over the 26-week treatment period, rising from a median of 0.07 mmol/L at baseline to 0.10 mmol/L, while BHB levels in the placebo group declined slightly (p = 0.002). Although these absolute values remain within the normal physiological range, even small, chronic increases in BHB can have meaningful biological effects.
BHB has been shown in prior studies to:
- Reduce oxidative stress by inhibiting class I and II histone deacetylases (HDACs), thereby enhancing the expression of antioxidant genes.
- Support mitochondrial respiration, offering a more energetically efficient fuel source than glucose while generating fewer reactive oxygen species.
- Lower inflammation by inhibiting the NLRP3 inflammasome, a key driver of age-related chronic inflammation.
- Enhance stress-response pathways, including FOXO transcription factors and SIRT3-mediated mitochondrial resilience.
- Promote vascular and neuronal protection, with some animal studies suggesting BHB may help counteract age-related cognitive decline.
These effects overlap closely with the molecular benefits of fasting and caloric restriction, which is one reason BHB is increasingly viewed as an endogenous anti-aging metabolite. SGLT2 inhibitors, by shifting cellular energy reliance from glucose toward fat oxidation, generate a steady, mild elevation in BHB that resembles aspects of nutritional ketosis—but without requiring long-term dietary restriction.
Importantly, in this trial, the increase in BHB did not coincide with elevations in inflammatory cytokines or signals of ketoacidosis. No cases of diabetic ketoacidosis were detected, and metabolic parameters remained stable or improved. This is consistent with the broader clinical literature: when used at therapeutic doses, SGLT2 inhibitors typically increase ketone levels modestly and safely.
Taken together, the rise in BHB provides another mechanistic link between henagliflozin and cellular resilience. By supplying a cleaner mitochondrial fuel, reducing inflammatory signaling, and activating protective stress-response pathways, BHB may contribute to the improvements seen across telomere preservation, immune function, and metabolic health. While more research is needed to determine whether chronic BHB elevation directly slows biological aging in humans, the metabolite’s profile fits the broader pattern emerging in this trial: SGLT2 inhibition appears to shift the body toward a metabolic state that supports healthier aging.
Metabolic Benefits That Support Cellular Longevity
As anticipated for an SGLT2 inhibitor, henagliflozin produced substantial improvements in metabolic health, and these shifts are directly relevant to aging biology. Elevated glucose, excess body fat, and high uric acid all contribute to oxidative stress, mitochondrial dysfunction, and chronic inflammation, cellular stressors that accelerate telomere erosion and impair tissue repair. By reducing these metabolic burdens, the drug created a physiological environment that supports healthier cellular aging. [3]
After 26 weeks of treatment, the henagliflozin group showed consistently greater improvements than placebo:
- Hemoglobin A1c (HbA1c), a measure of average glucose over three months, fell by −1.37% versus −1.04%.
- Fasting plasma glucose (FPG) decreased by −1.92 mmol/L compared with −1.17 mmol/L.
- Body mass index (BMI) declined by −1.32 points, versus −0.81 in the placebo arm (p ≈ 0.008).
- Average weight loss reached −3.55 kg on henagliflozin, compared with −2.23 kg with placebo.
- Serum uric acid, a marker tied to inflammation, vascular aging, and oxidative DNA damage, dropped substantially (−48.9 μmol/L vs −10.7 μmol/L)
These improvements extend beyond routine diabetes management. Lower glucose reduces glycation, the damaging reaction between sugars and proteins or DNA. Better insulin sensitivity stabilizes mitochondrial energy production, weight loss decreases inflammatory signaling from adipose tissue, and lower uric acid reflects reduced oxidative load. [1]
The Metabolic Reprogramming Behind Henagliflozin’s Anti-Aging Signals
To understand how henagliflozin might influence aging biology beyond glucose control, the researchers analyzed blood samples using metabolomics. This technique measures hundreds of small molecules produced by the body as it processes energy, fats, and amino acids. This gives a snapshot of what is happening inside cells at a biochemical level. Fifty-six participants were included in this part of the study (32 on henagliflozin and 22 on placebo). [1]
After 26 weeks, 53 metabolites differed significantly between the two groups. When viewed together, these changes formed a pattern that closely resembles what is seen during caloric restriction, a state known to support healthier aging in many organisms. [1]
One key signal was an increase in thiamine (vitamin B1). Thiamine is essential for several enzyme systems inside mitochondria, the structures that generate most of the cell’s energy. Higher thiamine availability generally means that mitochondria can run their energy-producing reactions more efficiently and with less strain. This is important because mitochondrial fatigue and inefficient energy production are central features of cellular aging. [1]
The second major pattern was a decline in certain stress-associated lipids, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingosine. These lipids tend to rise when cells experience metabolic strain, membrane instability, or chronic inflammation. Their reduction in the henagliflozin group suggests a “cleaner,” lower-stress lipid environment.
Several of these shifts have additional significance:
- PC and PE are major phospholipids that support membrane structure. When elevated, they can reflect membrane remodeling under stress — their decline here hints at reduced cellular strain and improved membrane stability.
- Sphingosine, a bioactive sphingolipid, is particularly notable. Elevated sphingosine levels are associated with worsening inflammation, impaired immune regulation, and neurodegenerative processes — including pathways implicated in Alzheimer’s disease. Lower sphingosine levels in this trial suggest a shift toward a less inflammatory, more neuroprotective biochemical state.
When these findings are combined, they form a caloric-restriction–mimetic profile: more mitochondrial support, less lipid-related stress, and improved biochemical efficiency. In other words, henagliflozin appears to trigger some of the same molecular adjustments the body makes during fasting: better energy handling, reduced metabolic noise, and enhanced cellular resilience.[1]
This metabolic signature helps explain why downstream aging markers in the study, such as telomere preservation and improved immune-cell function, also moved in a favorable direction. It suggests that SGLT2 inhibitors may act as nutrient-sensing modulators, influencing not only glucose levels but the broader biochemical environment that shapes how cells age.
What This Study Reveals About Targeting Metabolism to Slow Aging
When viewed as a whole, the results of the Zhang et al. trial tell a coherent story. Several biological systems—chromosomes, hormones, immune cells, and core metabolic pathways —shifted in a direction associated with healthier aging. Telomeres became longer instead of shorter. Growth-factor signals that push cells toward rapid growth are dialed down. The body produced slightly more ketones, suggesting a shift toward fat-burning. Immune cells responsible for clearing damaged or dysfunctional cells became more active. And the metabolomics data showed a pattern consistent with more efficient energy use and less cellular stress. Each of these changes has been linked to better aging in laboratory studies, but this is one of the first times they have been seen together in a human drug trial. [1]
The underlying biology makes sense. SGLT2 inhibitors cause the kidneys to excrete extra glucose into the urine, creating a small, steady energy deficit. The body responds by switching to backup fuel sources, especially fat, and by activating many of the same pathways that turn on during fasting. These include well-known “stress-resilience” systems inside cells that help repair damage, improve mitochondrial function, and make energy use more efficient. This shift away from constant growth and toward maintenance is a pattern seen in many species that age more slowly. [2]
That is what makes this study important. It is the first randomized, double-blind, placebo-controlled trial specifically designed to examine whether an SGLT2 inhibitor can influence biomarkers directly related to aging biology. The fact that multiple, largely independent markers—telomeres, IGFBP-3, CTL granzyme B, BHB levels, and metabolomic pathways—moved in a convergent direction suggests that the telomere findings were not an isolated anomaly but part of a broader physiological shift. While none of the individual results alone would be sufficient to claim a slowing of aging, the composite picture supports a mechanistically plausible pattern consistent with improved cellular resilience.
At the same time, the findings should be interpreted with appropriate scientific caution. The intervention lasted only 26 weeks, which is a short window for assessing any aspect of human aging. Telomere length, while informative, is still a proxy marker rather than a validated predictor of lifespan or long-term health outcomes. The immune and metabolomics analyses were conducted in relatively small subgroups, increasing the potential for variability. Because all participants had type 2 diabetes—a condition associated with accelerated biological aging—it remains unknown whether similar shifts would appear in younger individuals or in those without metabolic disease. Another key question is whether these effects persist after discontinuation of therapy or require ongoing treatment to maintain.
It is also important to acknowledge that henagliflozin itself is not yet approved outside Asia. However, its mechanism is shared across the SGLT2 inhibitor class. Drugs widely available in the U.S. and Europe—such as empagliflozin, dapagliflozin, and bexagliflozin—operate through the same core biology, making it plausible that similar molecular effects could be observed with these agents, even if this remains to be formally tested.
With all that said, the trial marks a meaningful step forward. It places SGLT2 inhibitors among the very small number of drug classes with randomized human evidence suggesting beneficial effects across multiple hallmarks of aging. It reinforces the idea that aging is deeply intertwined with metabolic physiology. And it provides an early proof-of-concept that shifting the body’s energy state—even modestly—may influence the molecular processes that shape how we age. While larger and longer trials will be essential, this study advances the possibility that widely used metabolic drugs could play a role in promoting healthier aging.
- Zhang J, Cai W, Liu D, et al. Effect of henagliflozin on aging biomarkers in patients with type 2 diabetes: A multicenter, randomized, double-blind, placebo-controlled study. Cell Reports Medicine. 2025;6(9):102331. doi:10.1016/j.xcrm.2025.102331.
- Seidu S, Alabraba V, Davies S, et al. SGLT2 inhibitors – the new standard of care for cardiovascular, renal and metabolic protection in type 2 diabetes: A narrative review. Diabetes Therapy. 2024;15:1099–1124. doi:10.1007/s13300-024-01550-5.
- Chakravarti D, LaBella KA, DePinho RA. Telomeres: History, health, and hallmarks of aging. Cell. 2021;184(2):306–322. doi:10.1016/j.cell.2020.12.028.
- Schellnegger M, Hofmann E, Carnieletto M, Kamolz L-P. Unlocking longevity: The role of telomeres and their targeting interventions. Frontiers in Aging. 2024;5:1339317. doi:10.3389/fragi.2024.1339317.
Related studies